Grover A, Mancini E, Moore S, Mead AJ, Atkinson D, Rasmussen KD, O’Carroll D, Jacobsen SEW, Nerlov C. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J Exp Med 2014;211: 181-188.
Prepared by: Kushkaran Kaur, Fall 2014
Layman’s Summary
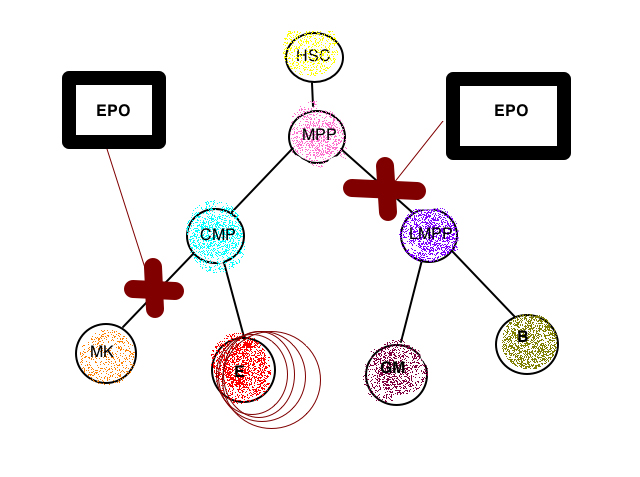
Hematopoietic stem cells (HSCs) are found in the bone marrow and give rise to myeloid and lymphoid blood cells through a process of differentiation. Red blood cells, a.k.a. erythrocytes, come from the myeloid progenitor cells. Progenitor cells are cells that differentiate into other types of cells. Erythropoietin (Epo) is an erythroid stress cytokine, which helps in the development of committed erythroid progenitors. During physiological emergencies, such as anemia, the levels of erythropoietin can increase in order to elevate the production of certain cell types. The researchers of this experiment demonstrate the ability of Epo to suppress the differentiation of HSCs into any cells other than erythroid cells.
Experiments were done in mice (in vivo) and in test tubes (in vitro) to see the effects of Epo on colonies of cells and the development of cells along a lineage. They concluded from the experiments that Epo increases the development of erythroid cells and decreases the development of other cells. Lineage reprogramming favors production of erythroid cells over other cell types. Pharmacological interventions suggested signaling pathways involving enzymes PI3 kinase and Erk.
The researchers concluded that high levels of Epo, associated with severe anemia, acted on HSCs to reduce the number of lymphoid cells and helped in the development of erythroid cells. This is the body’s response to the low number of erythroid cells in the state of anemia.
The research was extensive and looked at many aspects of how Epo can take effect at different sections of the HSC lineage. However, it is unclear exactly how many experiments were conducted and how exactly test subjects were selected for the analyses. Also, the paper referred to unpublished research in key points. The experiments raised a question as to whether suppression of lymphoid cell development will cause a weak immune system prone to common infections and diseases. Furthermore, the suppression of platelets may cause complications such as bleeding. More trials and details about experimental method may help these results to be used in the search for a better treatment for conditions such as severe anemia.
Scientific Summary
Erythropoietin is an erythroid stress cytokine that is involved in the development of committed erythroid progenitors. Studies have shown that cytokines promote the survival and expansion of committed progenitors during hematopoiesis. However, the information remains unclear on the upstream effects of erythropoietin on multipotent cells. During physiological emergencies, such as anemia, the levels of erythropoietin and myeloid colony stimulating factors can be increased to elevate the production of certain cell types. The Epo-Epo receptor signaling pathway is important for proliferation and survival of erythroid (E) progenitors. Multipotent hematopoietic stem cells express a functional Epo receptor but the effect of high levels of Epo on non-erythroid hematopoietic lineages and multipotent stem cells and progenitors is unclear. Researchers of this paper observed that high levels of systemic Epo reprogram transcriptosomes of multi- and bi-potent hematopoietic stem cell progenitors in vivo. This induces a lineage bias at all bifurcations of the lineage leading to an increase in erythroid output and a decrease in myeloid output. Therefore, the authors proposed that Epo has a role in vivo to suppress non-erythroid fates of cells, showcasing the ability of Epo as a cytokine to bias successive lineages in favor of erythroid cells.
The elevation of systemic Epo increased peripheral blood erythrocytes. Population of Lin- Sca-1- c-Kit+ cells showed an increase in erythroid colonies and progenitors in the presence of Epo and a decrease in all other colonies and progenitors. Decrease in Flt3 expression in the cells showed that Epo impaired the formation of any non-erythroid progenitors and promoted the formation of erythroid progenitors within the HSC/MPP compartment. Existing global gene profiling data showed up-regulation of genes with E-lineage commitment and down regulation of genes associated with the other myeloid progenitors. This suggested lineage programming could be regulated by Epo. Transplantation of cells with Gata 1-deficiency and wild-type cells into irradiated mice showed endogenous erythropoietic stress caused transcriptional HSC reprogramming. EpoR signaling, through PI3 kinase activation, upregulated preCFU-E genes whereas, JAK/STAT and Erk inhibition converted the normal inhibition by Epo to robust up-regulation. Epo-induced reprogramming of HSCs caused an increase in number of erythrocytes and a decrease in myeloid cells.
The results indicated that elevated Epo levels suppressed the levels of phenotypic and functional non-erythroid progenitors in the bone marrow and, transplantation of Epo exposed HSCs, resulting in an erythroid bias of the lineage. The research was extensive and looked at many aspects of how Epo can take effect at different parts of the HSC lineage. At times, it was difficult to appreciate the findings due to low statistical numbers, and reference to unpublished research in key points. The experiments raised a question as to whether suppression of lymphoid cell development will cause a weak immune system prone to common infections and diseases. Furthermore, the suppression of platelets may cause complications such as bleeding. More trials and details about experimental method may help this research be used to find a better treatment for conditions such as severe anemia.
|
