Summarized by: Louis Espinosa and Nadia Senmartin, Fall
2007
LAY REVIEW
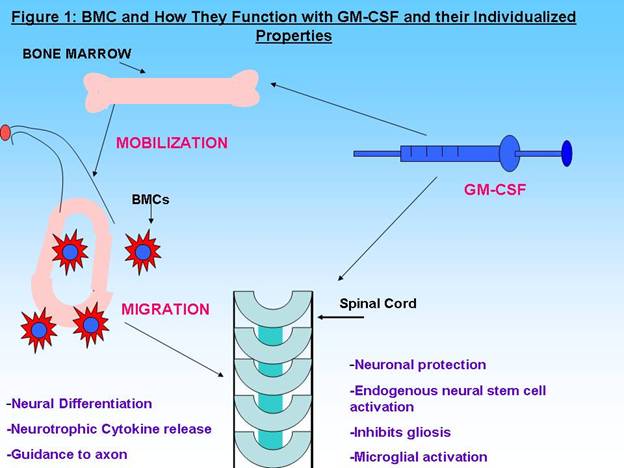
Spinal Cord injury (SCI) patients presently have no curative form of
treatments. The lack of treatments is mainly due to the seeming inability of
the Spinal Cord to regenerate after trauma. Previous experiments have deduced
the possibility of Bone Marrow Cell transplantation as a prospect for
therapeutic intervention after SCI. The use of bone marrow harvested from the
same patient shows a glimpse of hope since these cells have been shown to revive
some of the cells which could in turn stimulate the re-growth of the spinal cord
[2]. This re-growth could lead to functional improvement, although the progress
may vary without a cure [Figure 1].
This summary is based on a paper in which the authors previously used only
bone marrow cells without other treatments. The researchers postulated that
combining treatment with bone marrow cells and the growth factor, GM-CSF
(Granulocyte Macrophage Colony stimulating factor) would lead to a better
outcome [3]. GM-CSF has been shown to decrease cell death in the nervous
system, suggesting its potential to rescue cells destined for demise due to
injury. Thus, the scientists premise that combined therapy with bone marrow
cells and GM-CSF would enhance the outcome for SCI repair [4].
Phase I/II Clinical trials were conducted to define the proper dosing,
identify side effects, and provide preliminary information on the potential of
this new treatment, and also to get information on the safety and benefit.
The patients were divided into 3 groups and a control group. The
experimental groups were based on the time between injury and transplantation as
follows:
Acute: <2 weeks
Subacute: 2-8 weeks
Chronic: >8 weeks.
Control: No treatment
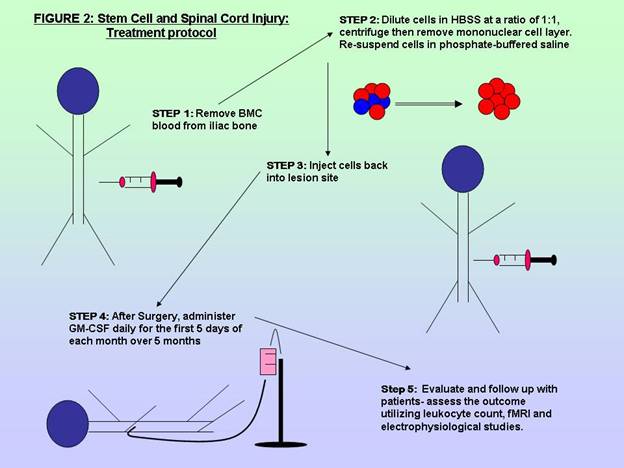
All patients were treated with decompression surgery of the spinal canal and
the three treatment groups were also administered the treatment protocol, which
entailed removing bone marrow cells, centrifuging and re-suspending the cells
and then re-injecting into the patient [1]. After surgery, a regimen of 5 cycles
of GM-CSF was administered, daily for the first 5 days of each month, over 5
months. [Figure 2]
The patients were examined for an average of 10.4 months. The evaluation
process consisted of total white blood cell counts, MRI and electrophysiological
studies for neuronal functions.
Overall, 42.9% of patients in the
treatment group showed an increase in the diameter of the spinal cord at the
cell transplantation site, but 33.3% of patients showed a decrease in diameter
at the transplantation site while 28.6% showed evidence of spinal cord
enhancement [1].
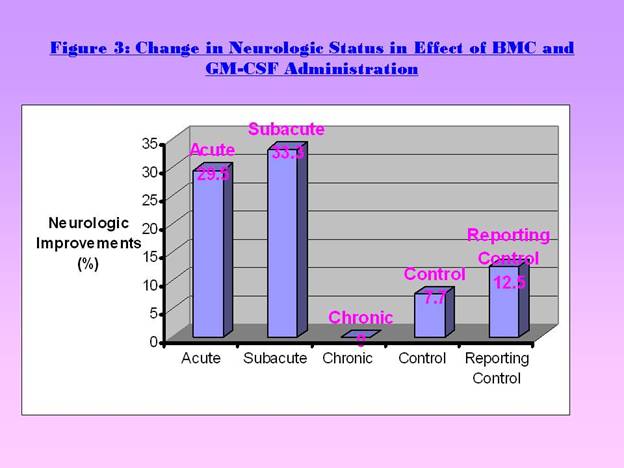
Acute group: 29.5% showed neurological improvement (Figure 3).
Subacute group: 33.3% showed changes (Figure 3).
Chronic group: No evidence of neurological changes [1] (Figure 3).
There was no considerable change, including tumor formation caused by the
implanted cells. Overall, neuropathic or chronic pain that occured due to damage
or pathological changes of the nervous system occurred in 20% of patients within
the treatment group. This indicated that the treatment may be associated with an
increased risk of developing neuropathic pain. However, the overall the side
effects were considered minimal and they did not inhibit the ongoing trial.
The present study demonstrated that utilizing BMC in conjunction with GM-CSF
seems to be beneficial and the minimal side effects were insufficient to
invalidate the trial. However, the study is associated with caveats. Firstly,
autologous BMC, which were used to prevent rejection, contained mixed cell
populations. Thus, it is unclear which cell subsets were important and whether
other cells could be the cause of the side effects. Secondly, future expanded
studies might dissect the effects of age. Thirdly, longer follow-up times are
needed to fully evaluate the outcome, including untoward effects.
This Phase I/II clinical trial did indeed provide a platform for further
investigations. It proved that there were no significant side effects of
administering the protocol and it allows for another alternative for SCI
patients. The researchers have established that there is a greater possible
enhancement in administrating their protocol as compared to no treatment. This
study could form the basis for future studies in cell-based therapies for SCI
patients.
References
- Yoon SH, Yu SS, Young HP et al. Complete Spinal Cord Injury Treatment Using
Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation With
Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells 2007;25:2066 -73.
- Koshizuka S, Okada S, Okawa A et al. Transplanted hematopoietic stem cells
from bone marrow differentiate into neural lineage cells and promote functional
recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 2004;63:64-72.
- Park HC, Shim YS, Ha Y et al. Treatment of complete spinal cord injury
patients by autologous bone marrow cell transplantation and administration of
granulocyte-macrophage colony stimulating factor. Tissue Eng 2005;11:913-22.
- Ha Y, Kim YS, Cho JM et al. Granulocyte macrophage colony stimulating factor
(GM-CSF) prevents apoptosis and improves functional outcome in experimental
spinal cord contusion injury. J Neurosurg 2005;11:55– 61.
SCIENTIFIC SUMMARY
The yearly incidence of Traumatic Spinal Cord Injury is predicted to exceed
13,000 people by the year 2010 with an overall population of 247,000 in the
United States alone (1,2). This despite ever improving safety equipment and
continually revised regulation in the areas of work and transportation safety.
Many options have been explored for treatment but the end result is usually loss
of neural function due to spinal cord injury which will mean a lifetime of
debilitation. The long standing theory is that once the CNS neural cell has
been damaged there is no repair.
Several in vivo and in vitro with mesenchymal stem cells
and hematopoietic stem cells have revealed the possibility for cell therapy in
spinal cord injury. The different techniques showed improved functions, neuronal
protection with decreased glial scars (3-5). In addition to stem cells, research
studies have also incorporated different cytokines as therapeutic agents for
spinal cord injury. These studies have yielded evidence of neuronal protection
from apoptosis and the stimulation of axonal regeneration from the
administration of GM-CSF (5). The review focused on the combination of these two
forms of therapies in phaseI/II trials in humans (7). Similar trials were done
with limited number of patients and favorable results. (6) The researchers
implanted bone marrow cells directly at the site of injury and administered a
GM-CSF therapy regime in order to establish the safety of this treatment and its
potential effects in human subjects.
The subjects selected for this trial were all assessed as being in the
American Spinal Injury Association Impairment Scale grade A. This would mean
that these patients had no sensory or motor function below the level of the
injury. The injury in these patients was one of contusion not transection.
Therefore, subjects with transection of the spinal cord, or additional medical
issues such as need for a ventilator, pre-existing medical condition, and/or
fever were excluded.
The subject pool was divided into a treatment and control pool. The subjects
all received decompression and fusion treatment for their injuries. The
treatment group was broken into three categories based on the time interval
between injury and therapy in order to establish the ideal window for this
strategy. The categories were as follows:
10-14 days post-injury
2 weeks to 8 weeks post-injury
> 8 weeks post-injury
Base line scans and functional assessments were recorded for both control and
treatment groups. The treatment group had bone marrow cells harvested from
their iliac bone, processed, and implanted at the site of injury. The treatment
subjects would then receive five doses of GM-CSF for the first five days of the
month repeated for five months. Mean follow-up and evaluation period of the
subjects was 10.4 months. The follow-up consisted of fMRI, neurological
assessment, and electrophysiological studies.
The treatment group fared well with regard to the safety of the treatment
strategy. There were no infections, obvious tumors, or severe complications as
a result of the study. Issues identified were an elevated number of subjects
experiencing neuropathic pain as compared to the control group. One detail of
interest in regards to neuropathic pain is the prevalence of the neuropathic
pain in the groups that received the therapy further out from the original
injury. One other issue of note was the possible generation of aberrant neurons
in one case as seen in the fMRI. The subject presented activity in areas not
associated with a normal response to the proprioceptive testing being done.
Discussion
This trial presented evidence of the safety of
this therapy strategy in patients with spinal cord injury. There were no severe
complications from the surgery or the administration of the GM-CSF other than
some fever and the increase in neuropathic pain. Both of these effects were
expected from the administration of the GM-CSF. There were some positive
results in the trial, namely an increase in neurological function in the acute
and sub-acute groups versus the control, though the chronic group did not
demonstrate any increase in function.
Some questions and suggestions do present themselves after a review of the
study. The first deals with the use of bone marrow cells. The intent was to
utilize autologous cells in order to circumvent any immune response and ethical
considerations. The method used to harvest the cells was under general
anesthetic and performed in conjunction with the implantation of the cells. This
required keeping the patient under anesthetic and on the operating table for
approximately four hours. Though this may involve less risk to the patient than
having performed two separate procedures (one to harvest and one to implant) it
still creates the issue of time. The bone marrow cells were harvested,
centrifuged, and washed while the patient was under. The narrow time window did
not allow the harvest to be run through a flow cytometry device or some other
method which would allow the researchers to target the specific type of cells
that they wanted to implant. As a result the effect of some of the inflammatory
agents and immune cells found in the bone marrow cells come into play in this
study. The inability to target specific stem cell types opens the opportunity
for the implantation to incorporate antagonists to the desired repair. There
was some evidence of enlargement of the spinal cord though no evidence of
tumors. The qualifier is that a ten month follow-up may not have been long
enough to present a tumor. A future study could incorporate a peripheral blood
harvest using a drug similar to Neupogen. This drug mobilizes stem cells into
the peripheral blood for harvest without requiring the subject to have both the
harvest and implantation done in one procedure. This would allow the luxury of
time for the manipulation of the autologous cells in order to target specific
stem cell types. There is some controversy over whether this type of treatment
can result in inducing cancer patients. Further studies must be done along with
careful monitoring of long-term studies, along with informing potential
recipients of the risks. (8-10) There is also indication that stem cells
harvested from the peripheral blood are in a more undifferentiated state than
those taken directly from the iliac bone.(11) This would allow cleaner
indications of the efficacy of stem cells in the regeneration of CNS neural
cells. Separation of the GM-CSF treatment from that of the implantation through
use of another treatment group would also contribute to a clearer understanding
of the mechanisms involved.
Another issue was the age distribution of the different treatment groups.
Though understandable given the difficulty in finding willing subjects there is
an issue given that the sub-acute group was considerably younger than the acute
and chronic groups. This younger group demonstrated the greatest increase in
neurologic function but it may have been due to the younger body having a
greater capacity to recover.
Lastly, this study was conducted in one location. Though efforts were made
to blind the neurological assessors to the identity of the patients there still
may have been some prejudice. The authors of the study suggest that further
research would require the involvement of multiple facilities in order to
generate a truly randomized and blinded study.
This work presents another step in the direction of a solution for people
with spinal cord injuries. Some work must be done in the refinement of
techniques in order to minimize the risk to the patient and clarify the
cause/effect of implanting specific cell types and the use of cytokine therapy
in these patients. These steps can lead to a viable solution with clearly
defined goals and effectors.
References
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2-S12
- Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62-8.
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001-5.
- Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623-30.
- Ha Y, Park HS, Park CW, Yoon SH, Park SR, Hyun DK, Kim EY, Park HC. Synthes Award for Resident Research on Spinal Cord and Spinal Column Injury: granulocyte macrophage colony stimulating factor (GM-CSF) prevents apoptosis and improves functional outcome in experimental spinal cord contusion injury. Clin Neurosurg. 2005;52:341-347.
- Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Engineering. 2005;11:913-22.
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25: 2066-73.
- http://www.neupogen.com/pi.html
- Confer DL, Miller JP. Long-term safety of filgrastim (rhG-CSF) administration. Br J Haematol. 2007;137:76-80
- Bennett CL, Evens AM, Andritsos LA, Balasubramanian L, Mai M, Fisher MJ, Kuzel TM, Angelotta C, McKoy JM, Vose JM, Bierman PJ, Kuter DJ, Trifilio SM, Devine SM, Tallman MS. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the Research on Adverse Drug Events and Reports (RADAR) project. Br J Haematol. 2006;135:642-50.
- Hassan HT, Zeller W, Stockschläder M, Krüger W, Hoffknecht MM, Zander AR. Comparison between bone marrow and G-CSF-mobilized peripheral blood allografts undergoing clinical scale CD34+ cell selection. Stem Cells. 1996;14:419-29.
- Pelus LM, Fukuda S. 2007. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. (In press).
