(Back)
Thalidomide corrects impaired mesenchymal stem cell function in inducing tolerogenic DCs in patients with immune thrombocytopenia
AUTHORS: Ji Ma, Yun-na Ning, Miao Xu, Yu Hou, Ning Wang, Xiao-yan Hou, Ying-yi Yu, Hui Li, Wei-dong He, Lin=lin Shao, Hai Zhou, Ya-nan Min, Xin-guang Liu, Yan Shi, Ping Qin, Cheng-shan Guo, Ming Hou, and Jun Peng
REVIEWED BY: VANESSA PIZUTELLI, Fall 2014)
LAY SUMMARY
Multiple myeloma is an incurable condition in which genetically mutated plasma cells form tumors in bone and disrupt blood and platelet formation. These mutated plasma cells also release antibodies that cause organ failure. Multiple myeloma can be managed with chemotherapy and marrow replacement, however in rare cases these forms of treatment can lead to the development of an autoimmune disorder called immune thrombocytopenia (ITP). Patients with ITP produce antibodies that attack platelets and impair the patient’s ability to clot, thus causing excessive bleeding. These patients may also experience impaired activity and functionality of their mesenchymal stem cells (MSC), which are stem cells that have the potential to suppress the immune system. In this study, researchers investigated whether Thalidomide, a drug that alters the immune system, can correct MSC impairment in patients with ITP.
The authors confirmed that MSCs from ITP patients that have reduced function and have lost their ability to induce mature immune cells into ones that are less active, can be corrected with THD treatment and that modulatory effects of THD on MSC is caused by the regulation of cell signals. These findings are interesting because they show that THD can help lower the activity of one’s immune system. In patients with overactive immune systems, like patients with ITP, the reduced activity could mean less attack on platelets. However, more work must be done to support THD as a viable therapeutic agent for patients with ITP. The investigators failed to evaluate the relationship between THD and other immune cells, which may also be involved in the impaired functionality of MSC in patients with ITP. Furthermore, the authors failed to investigate the direct effect that THD treated MSCs have on platelets derived from patients with ITP, which would be the primary target in this auto-immune disorder. The authors should also consider designing an experiment that would help better define the role of MSCs in the development and perpetuation of ITP and whether or not restoring the functionality of these cells would improve the patient’s state of health. Finally, it is recommended that the authors attempt to investigate the therapeutic potential of THD in animal models with ITP-like symptoms to better understand this drug’s overall impact.
SCIENTIFIC SUMMARY
Thalidomide (THD) is a non-barbituate sedative hyponotic drug which has been used to treat patients with immune-mediated diseases. In patients with Immune Thrombocytopenia (ITP), THD serves as an immunomodulatory agent and helps manage the symptoms associated with this auto-immune disorder. Patients with ITP also experience decreased proliferative and functional capacity of their mesenchymal stem cells (MSC), which are involved in immune tolerance. In this study, researchers investigated the restorative potential of thalidomide (THD) on MSCs derived from ITP patients in vitro.
To determine the effect of THD on ITP derived MSCs, several assays were generated using primary ITP and MSCs from healthy patients. The proliferation assays revealed that THD has the potential to increase MSC proliferation in ITP patients (at a plasma concentration between 0.1-10 μg/mL) and reduce the rate of apoptosis in healthy- and ITP-derived MSC populations.
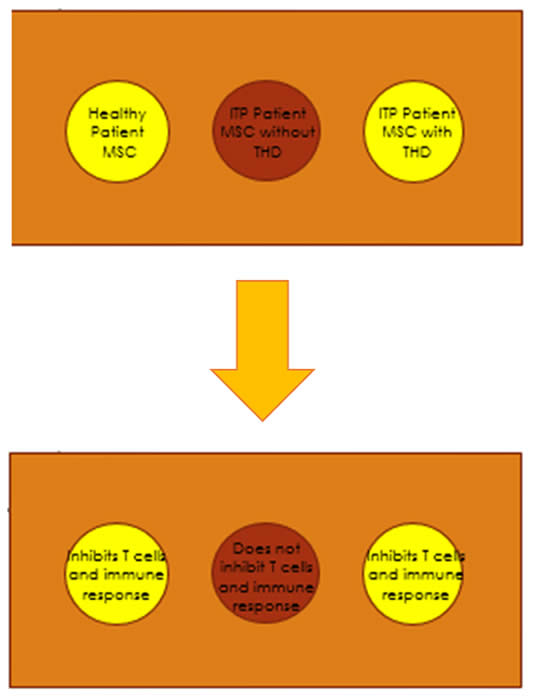
To confirm the loss of immunosuppressive ability of ITP-MSCs, CFSE-labeled CD4+ T cells were stimulated by allogeneic mDCs or PMA in the presence or absence of MSCs from healthy patients (control), MSCs from patients with ITP (MSC(ITP)), and THD-treated MSCs from ITP patients (THD-MSC (ITP)). When MSCs (control) or THD-MSCs (ITP) were added to mDC cocultures in the absence of PMA, they inhibited T cell proliferation, however the addition of MSCs (ITP) had no inhibitory effect and THD-MSCs (ITP) were unable to prevent T cell proliferation activated in the presence of PMA.
In assay measuring the levels of IL-4, IFN-γ, IDO, and IL-12, IL-4 and IDO were higher when DC4+ T cells and mDCs were cocultured with THD-MSCs while IFN-У levels were lower when cocultured with MSCs (ITP) or without MSCs. There was no significant difference in IL-12 level between cultures with THD-MSCs (ITP) or with MSCs (ITP), though it was reported that it was lower than without any MSCs in both cases. It was also found that mDCs regulated by MSCs from healthy controls suppressed the proliferation of T lymphocytes, whereas mDCs regulated by MSCs from ITP patients did not, and that the inhibitory capacity of mDCs was increased when incubated with THD-MSCs (ITP).
To investigate whether the inhibitory effect of tolerogenic DCs was strong enough to silence lymphocytes activated by PMA, mDCs regulated by MSCs from ITP patients, healthy controls, or THD-modulated MSCs were assayed. It was reported that these cells were unable to inhibit T-cell proliferation. Although tolerogenic mDCs could not prevent T cell proliferation activated by PMA, they did block the allogeneic mDC-activated T cell proliferation.
The authors also conducted phenotypic analysis of mDCs cocultured with MSCs from healthy controls, from ITP patients, and THD-modulated ITP MSCs and found that MSCs from ITP patients failed to downregulate CD80 and CD86 expression in mDCs, while MSCs from healthy controls significantly inhibit the expression of these costimulatory factors. THD-MSC(ITP) also downregulated expression of CD80 and CD86 in mDCs.
Since IL-10 and TGF-β1 are strong immunosuppressive cytokines secreted by antigen presenting cells, the researchers tested whether cytokines play a role in tolerogenic DC-mediated inhibition. They found that IL-10 and TGF-β1 of THD-MSCs (ITP) and mDCs were higher than mDCs alone or mDCs +MSCs (ITP). These results suggest that the MSC-derived inhibitory property of DCS is related to DC maturation state,TGF-β1 or IL-10 cytokine production, and that regulatory ability of MSCs from ITP patients could be regained upon THD modulation. After coculture with MSCs, the mDCs were able to differentiate into novel tolerogenic DC population and that acquisition of tolerance by mDCs upon incubation with MSCs depends on TIEG1.
Ultimately, the authors confirmed that MSCs from ITP patients that have reduced proliferative capacity and have lost ability to induce mature DCs (mDcs) into tolerance DCs, which can be corrected with THD treatment. They also concluded that modulatory effects of THD on MSC is caused by downreguation of caspase-8 and caspase-10 and upregulation Oct3/4 and tgf-β1. THD-modulated MSCs could regulate mDCs by downregulating the expression of costimulating factors and IL-12 expression by upregulation of IL-10, TGF-β1, and IDO expression, and that MSCs induced mDCs into TIEG1-dependent tolerogenic DCs.
While the authors of this study provide a general evaluation of the effects of THD on MSC, there is not enough evidence to support THD as a viable therapeutic agent for patients with ITP. The investigators failed to evaluate the relationship between THD and other antigen-presenting cells, which may also be involved in the impaired functionality of MSC in patients with ITP. Furthermore, the authors failed to investigate the direct effect THD-MSCs have on platelets derived from patients with ITP, which would be the primary target in this auto-immune disorder. The authors should also consider designing an experiment that would help better define MSCs role in the development and perpetuation of ITP and whether or not restoring the functionality of these cells would significantly improve the patient’s state of health. Also, they should consider using MSCs from non-primary ITP patients, as primary ITP MSCs may differ from secondary (acute or chronic) ITP cells. Finally, it is recommended that the authors attempt to investigate the therapeutic potential of THD in animal models with ITP-like symptoms to better understand this drug’s overall impact.
(Back)
|
