Koyanagi-Aoia M, Ohnukia M, Takahashia K, Okitaa K, Nomab H, Sawamuraa Y, Teramotoa I, Naritaa M, Sato Y, Ichisaka T, Amano N, Watanabea A, Morizane A, Yamada Y, Sato T, Takahashi J, Yamanaka S. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci 2014; 110:20569–20574.
Prepared by: Zachary Holseberg, Fall 2014
LAYMAN ABSTRACT
Pluripotent stem cells are primitive cells that can differentiate into any adult cell type. These cells have great potential for use in many applications, including regenerative medicine and drug discovery. Stem cells harvested from the inner cell mass of embryos (ESCs) have this potential, but there are many scientific, ethical and logistical concerns with the use of these cells. In 2006, Dr. Yamanaka of Kyoto University was the first to make pluripotent stem cells from fully differentiated adult cells. These cells, called induced pluripotent stem cells (iPSCs), share many qualities with embryonic stem cells. iPSCs are reprogrammed to become pluripotent by exposure to factors that control the expression of genes within the adult cell. These four genes, Oct4, Sox2, cMyc, and Klf4, are collectively referred to as Yamanaka factors.
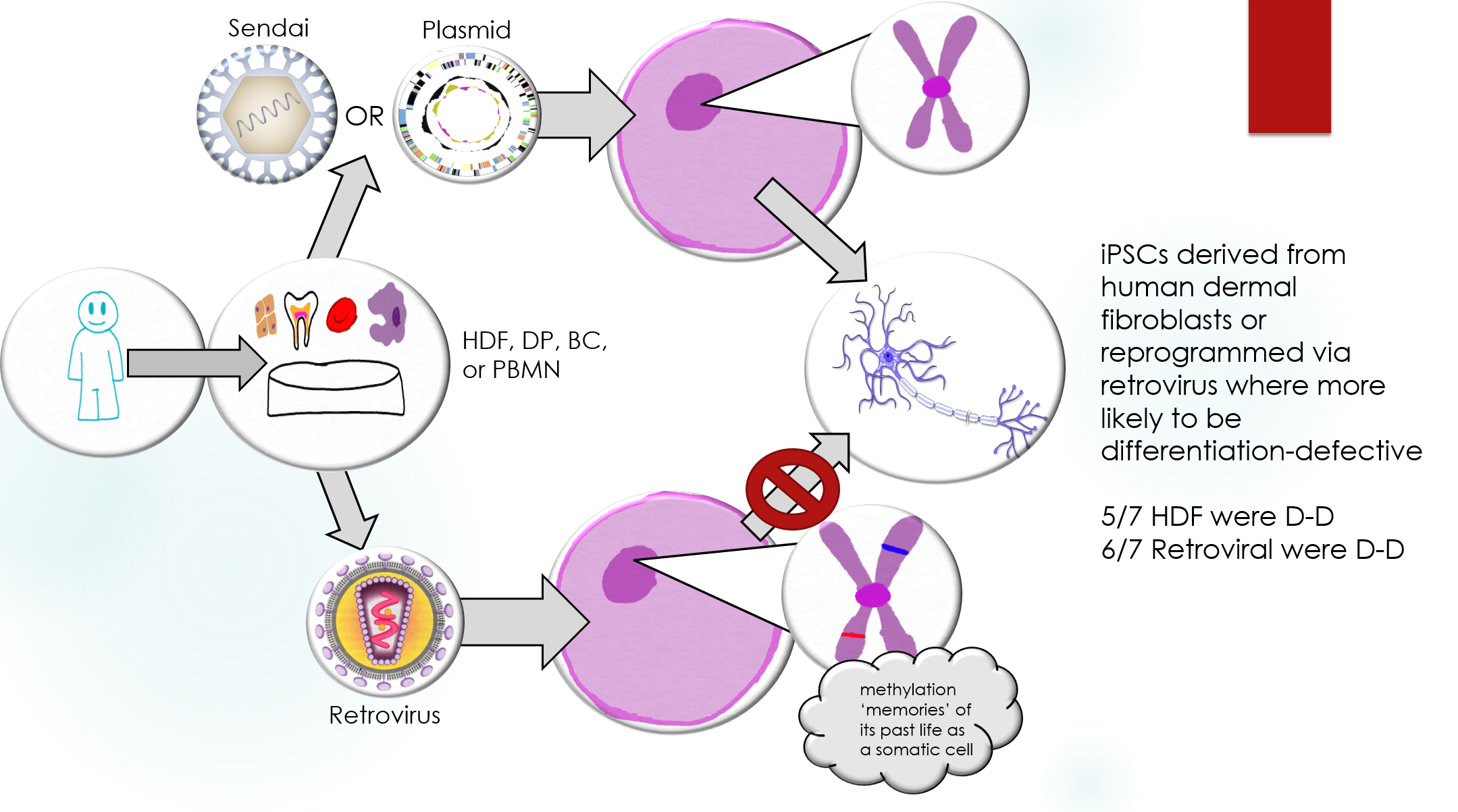
It is not yet fully understood if differences in iPSCs and ESCs may have clinical implications. This study aims to more fully characterize iPSCs and compare them to ESCs in both gene expression and capacity to differentiate into adult cells. Three different methods of generating iPSCs from four different adult cell types were examined and compared to ESCs. The results showed that reprogramming via retrovirus, which integrates into the host cell DNA, was more likely to cause iPSCs to become “differentiation-defective”. iPSCs produced using this method are less likely to differentiate into adult neurons when given the appropriate signals as compared to ESCs. Identifying the most effective method to produce these cells in important for future research.
The use of iPSCs presents many challenges that must be addressed before they can become viable candidates for clinical therapies. These cells may be more immediately valuable in the field of drug discovery, where they can be used as models for human disease. Cells that are difficult to gather from patients, such as heart and nervous cells, can be obtained from a skin sample. This can allow potential drugs to be screened for toxicity and effectiveness, potentially saving time and resources in drug development.
SCIENTIFIC ABSTRACT
The advent of induced pluripotent stem cells (iPSCs) offers a new method of producing pluripotent stem cells without the use of embryos. While iPSCs and ESCs share many characteristics, these is still controversy over whether they are distinct cell types. This study aims to characterize human (h) iPSCs and compare them to hESCs with regards to mRNA and miRNA levels, DNA methylation, and the capacity to differentiate. The hiPSCs were generated from human dermal fibroblasts (HDFs), dental-pulp stem cells (DP), cord blood cells (CB), and peripheral blood mononuclear cells (PBMN). Three methods of gene delivery were used: retroviruses, nonintegration episomal plasmids, and Sendai viruses.
The analyses of mRNA showed differences as overlaps between hiPSCs and hESCs. The analyses of miRNA and DNA methylation found no significant difference between the two cell types. The serum-free floating culture of embryoid body-like aggregates (SFEBq) method was used to induce neural differentiation in 40 hiPSC and 10 hESC lines. Over 80% differentiation efficiency was observed in both hiPSCs and hESCs as determined by the presence of polysialylated neural cell adhesion molecule (PSA-NCAM) neural cell marker. Some hiPSCs, however, had a higher proportion of OCT3/4+ cells, indicating a lower rate of differentiation. These clones were referred to as “differentiation-defective”. Global gene expression patterns of good and differentiation-defective hiPSCs were compared to identify a molecular fingerprint that could identify defective clones. Three genes: HHLA1, ABHD12, and C4orf51 were found to be up-regulated in defective hESCs as compared to good hiPSC and hESC lines. Differentiation-defective clones were also shown to form teratomas in SCID mice after in vitro neural directed differentiation. This highlights the need for genes in charge of differentiation-defectiveness in hiPSC lines to be further studied before possible applications in regenerative medicine.
In order to identify the best method for iPSC induction, the authors could have used a more diverse selection of somatic cell types and methods. Human dermal fibroblasts and the retroviral method produced the most defective cells, while peripheral blood mononuclear cells and Sendai virus produced the least defective cells. No HDFs were induced using Sendai virus, so it is unclear whether the cell origin or induction method is the major player in differentiation-defectiveness in hiPSCs. Producing iPSCs from each somatic cell type with each method would help provide further insight into the relationship between cell origin, method, and potential defectiveness.
|
