Stem cell research and regenerative medicine represent two of the most
exciting and potentially rewarding disciplines of biomedical science. This
resource serves as a reference for some of the recent findings, discoveries and
topics.
Current Topics - Last Three Years |
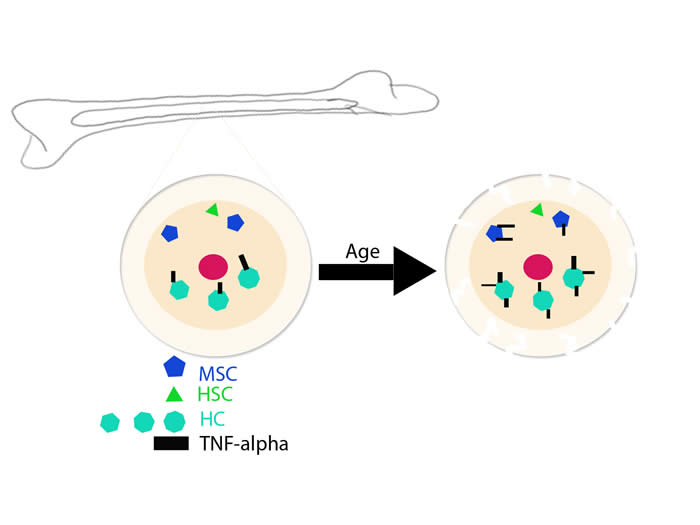 |
Paracrine effects of haematopoietic cells on human mesenchymal stem cells.
Studying the microenvironment of the bone marrow (BM) has revealed the relationship between hematopoietic (HSCs) and mesenchymal stem cells (MSCs). MSCs act as precursors to bone-forming and reabsorbing cells. It is known that MSCs also have a regulatory effect on the HSCs in the bone marrow when; however the inverse relationship remains unclear. The author hypothesizes that soluble proteins and small molecules released by hematopoietic cells are responsible for the regulation of MSC’s function in bone formation. In order to determine the validity of this hypothesis, a series of experiments were developed to grow both cell types within the same system and observe the growth and proliferation of osteoblasts (bone forming cells) and osteoclast (bone reabsorbing cells).
Click here for more information. |
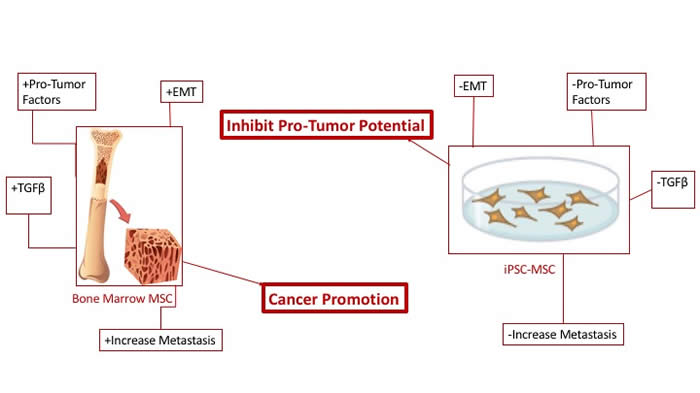 |
MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs.
Mesenchymal stem or stromal cells (MSCs) are multipotent cells that are capable of differentiating into a variety of cell types. MSCs can form connective tissues throughout the body and are capable of forming osteoblasts, fibroblasts, chondrocytes and adipocytes. MSCs have potential therapeutic applications in treating cancer and tissue regeneration due to cancer and radiation treatments. MSCs can serve as a vehicle for gene therapy for advance cancers, including treatment in bone, based on osteogenic properties. A contraindication for the use of bone marrow derived MSCs (BM-MSC) is based on the cells’s ability to promote tumor. This study asked if MSCs from transgene-free human induced pluripotent stem cells (iPSCs) can be a better alternative to BM-MSCs.
Click here for more information. |
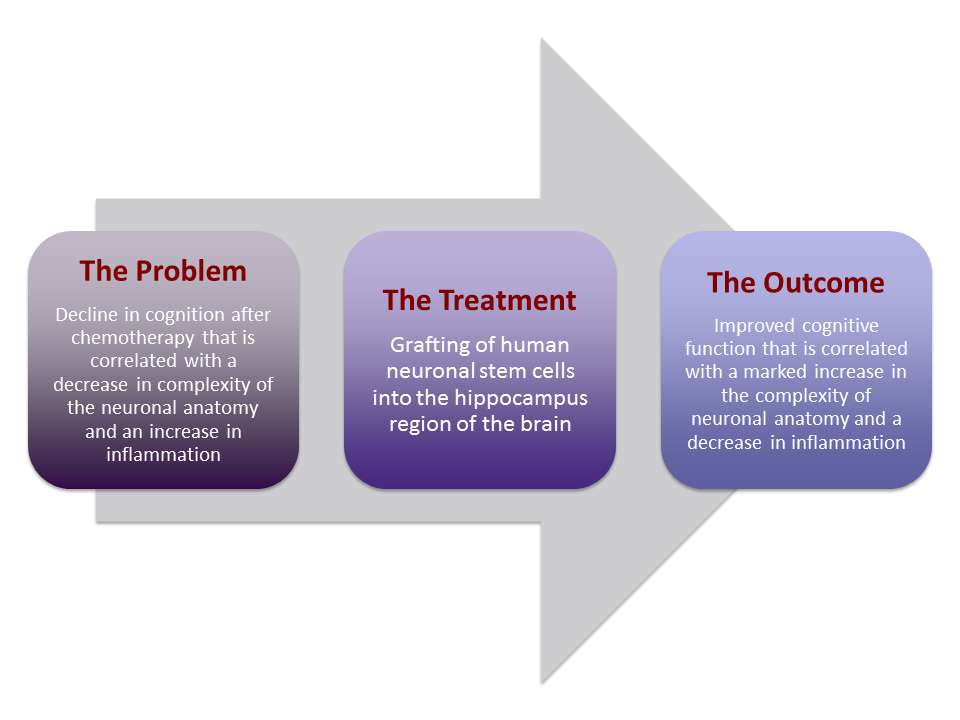 |
Stem Cell Transplantation Reverses Chemotherapy-Induced Cognitive Dysfunction.
Today’s oncological treatments have improved the outlook of survival for many advanced stage cancers however, cognitive decline is often noted as a side effect of chemotherapy, especially in late stage cancer patients. “Chemobrain” or “chemofog” is considered to be a result of brain cells being negatively affected by chemotherapy drugs. Patients tend to have slower processing speeds, problems with memory and difficulties paying attention. Prior research has shown that neurons in the hippocampus region of the brain (which controls memory formation and storage) are less complex in structure than their healthy counterparts. There is also indication of residual inflammation. Such damage is correlated with cognitive deterioration.
Click here for more information. |
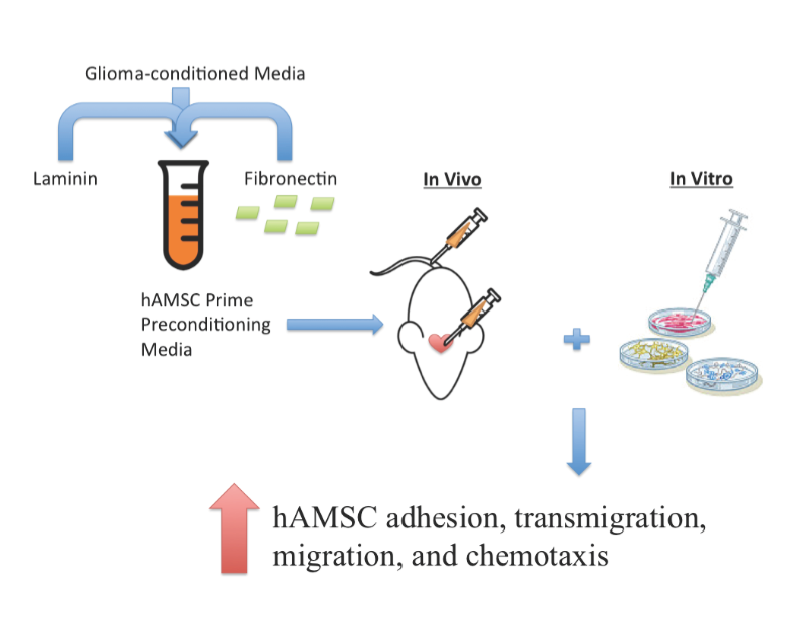 |
Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances
their homing to brain cancer.
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be found in several tissues such as bone marrow and adipose tissue. There is a growing interest to use MSCs for therapeutic delivery of drugs to treat neurological disorders. The attractiveness of using MSCs for such treatment are mostly due to the following: MSCs can home to sites of tissue injury; can cross the blood-brain barrier to sites of neurological, and do not provoke an immune response. In addition, unlike embryonic stem cells, MSCs are adult-derived, indicating reduced ethical concerns and ease in harvesting the source tissues. Unfortunately, MSCs have shown low therapeutic efficiency in treating neurological conditions because of their low numbers of engraftment (<1%) and their tendency to target healthy tissue rather than damaged tissue, resulting in unwanted side effects. Thus, the authors of the paper studied method to increase the therapeutic efficacy of MSCs on neurological disorders. The authors demonstrated that growth of human adipose-derived MSCs (hAMSCs), in the presence of key signaling proteins involved in MSC homing, enhanced the ability to home and integrate to sites of brain cancer.
Click here for more information. |
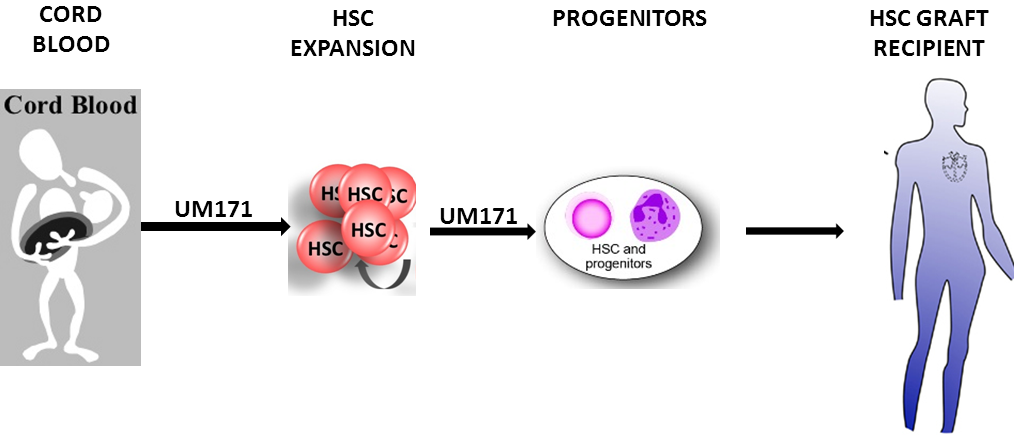 |
Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal.
Hematopoietic stem cell (HSC) transplant is the most viable option to curing hematological disorders. Currently, bone marrow HSCs are easily accessible for treatment. The compatibility of the HLA alleles between the donors and recipients predict the likelihood of immune rejection. Human cord blood (CB) has an immature immune system and a lower risk of chronic graft-versus-host disease. Unfortunately, CB extractions produce insufficient Hematopoietic progenitors (CD34+) cells to effectively treat adult patients. The researchers discovered that a small molecule, UM171, is capable of expanding the number and proliferative potential of CB derived LT-HSC. These researchers utilized mobilized peripheral blood (mPB) in a fed-batch system to screen 5280 drugs’ ability to expand CD34+CD45RA- cells. UM729 most significantly increased the number of CD34+CD45RA-. Furthermore, other drugs with increased expansion of CD34+CD45RA- targeted aryl hydrocarbon receptor (AhR) pathway.
Click here for more information.
|
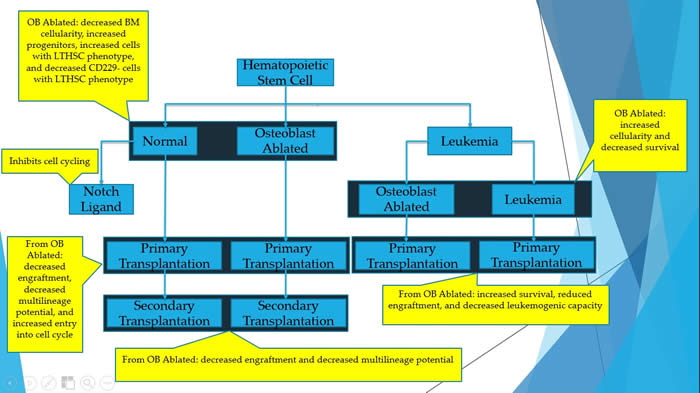 |
Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development.
The paper used a mouse model to activate chronic myelogenous leukemia. The authors were able to determine the effect that osteoblasts on normal and leukemic hematopoiesis. Osteoblasts are part of the bone marrow niche (microenvironment). Thus, these cells have a major role in controlling the fate of hematopoietic stem cells. This was proved by deleting the osteoblasts, followed by functional studies with normal and leukemic conditions. The deletion of osteoblasts resulted in decreased bone marrow cellularity, increased in hematopoietic stem and progenitor cells. Thus, it was concluded that osteoblasts are necessary for the regular development of hematopoiesis.
Click here for more information. |
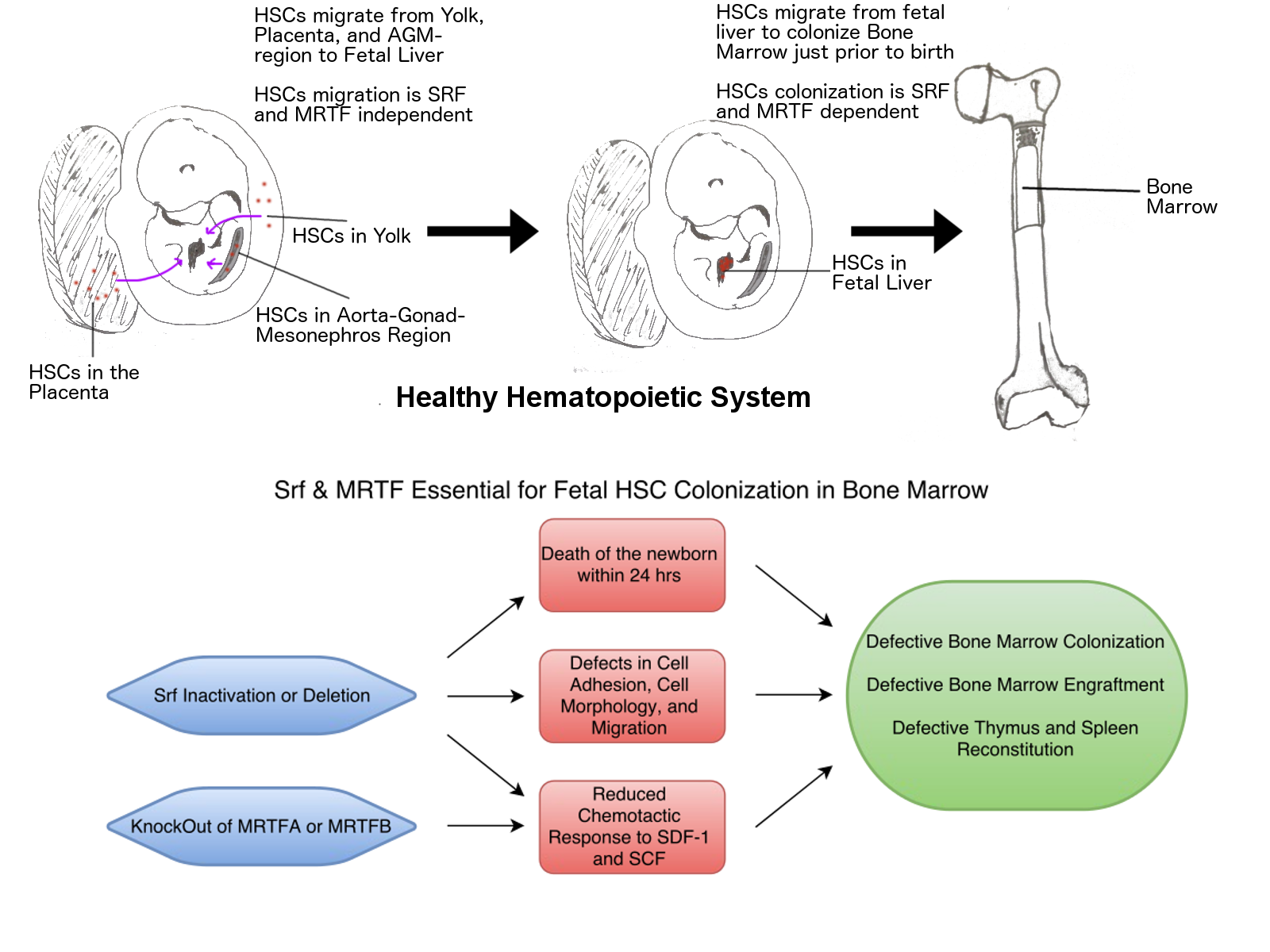 |
MRTF-SRF signaling is required for seeding of HSC/Ps in bone marrow during development.
The hematopoietic system constantly creates all of your blood cells over the course of your life. These blood cells all arise from hematopoietic stem cells (HSCs), which themselves reside in specialized stem-cell niches (a term for the specific microenvironment in the bone marrow that allows HSCs to thrive and renew). These cells do not start in these niches, though, in the early days of the fetus, these HSCs are initially generated in the regions of the fetus that would eventually become the torso and the yolk and placenta. They collect in the fetal liver, and finally colonize the bone marrow just prior to birth. Needless to say, if the HSCs do not become established in the bone marrow, the fetus will not survive.
Click here for more information. |
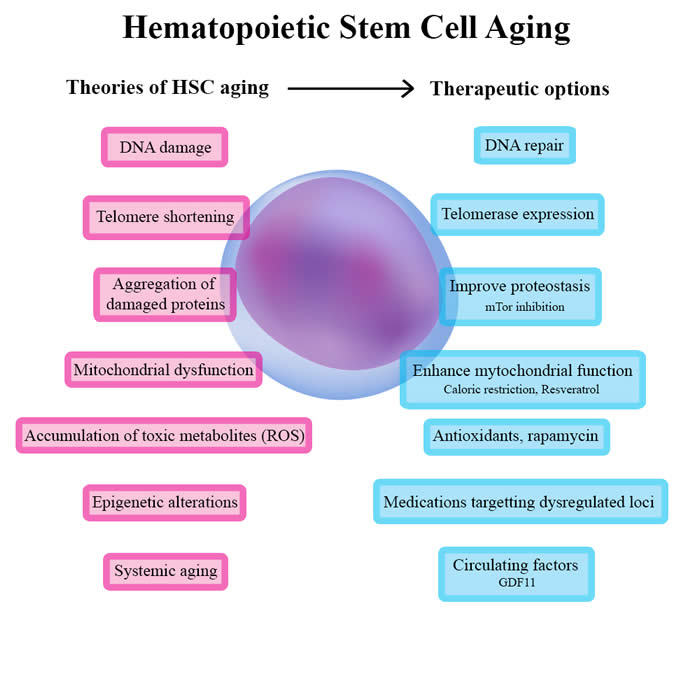 |
Concise review: Hematopoietic stem cell aging and the prospects for rejuvenation.
Aging is an inevitable phenomenon where our organism composed of multiple cells is submitted to progressive changes and eventually failure. It’s associated with the inability to maintain the balance in organs and tissues that are submitted to stress or injury. The resident stem cells in our body, the hematopoietic system that produces blood are also linked to the aging process. This results in a decrease in the adaptive immune response.
Click here for more information. |
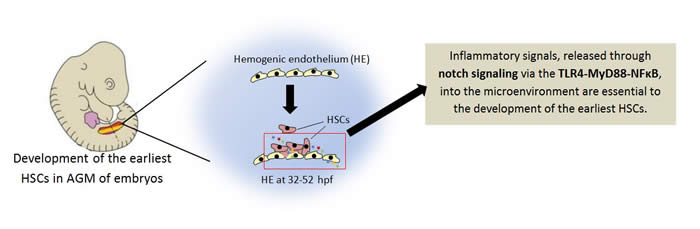 |
Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates.
The field of hematopoietic stem and progenitor cells (HSPCs) research has grown and expanded over the years. The findings in the field have played a major role in the discovery of treatment to blood-and hematopoietic -related cancers. Scientists are involved in research studies to understand how the earliest hematopoietic stem and progenitor cells are regulated. The emergence of the earliest HSPCs begins early the development of an embryo. The process occurs in the aorta-gonad-mesonephron (AGM) regions. The hemogenic endothelium begins to transform to hematopoietic stem and progenitor cells. Interestingly, the microenvironment or niche shows that hemogenic endothelium (HE) cells of the AGM release inflammatory signals, the same signals released when the immune system is combating an infection.
Click here for more information. |
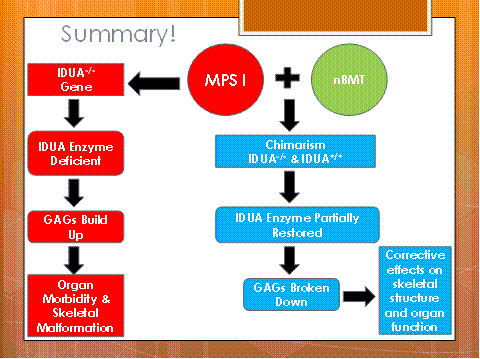 |
Neonatal Bone Marrow Transplantation: An Early Treatment Option for Mucopolysaccaridosis Type I Disease.
Muccopolysacchardosis type I (MPS I) is a genetic disorder which removes the body’s capability to break down certain types of sugars. When these sugars, termed glycosaminoglycans (GAGs), build up in the some of the cells, this can cause the bones and other muscular tissues, such as the heart, to become abnormally thicker than the normal tissues. These skeletal deformities can be very painful for patients with MPS I due to an early onset of carpal tunnel syndrome, arthritis, and stiffness as the bones become thick to occupy more space.
Click here for more information. |
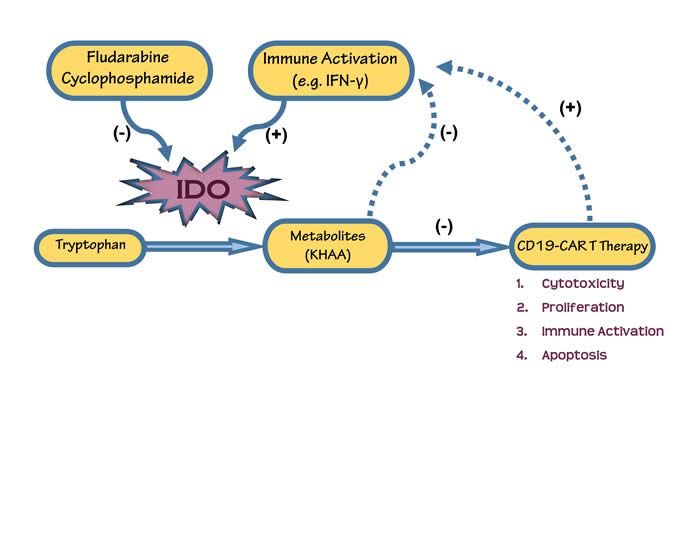 |
Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs.
Immunotherapy is a type of cancer treatment designed to boost the body’s natural defenses by helping the immune system work better at destroying cancer cells. Unfortunately, cancer cells develop mechanisms to fly under the radar of immune surveillance and evade detection. However, recent advancements in research have brought exciting developments in ‘targeted’ immunotherapies, that involve engineering/modifying a patient’s own immune cells to recognize and attack tumors. Engineered receptors are expressed on the surface of these T-cells which in turn provides them with the capability to identify specific proteins on cancer cells.
Click here for more information. |
|
Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells.
Pluripotent stem cells are primitive cells that can differentiate into any adult cell type. These cells have great potential for use in many applications, including regenerative medicine and drug discovery. Stem cells harvested from the inner cell mass of embryos (ESCs) have this potential, but there are many scientific, ethical and logistical concerns with the use of these cells. In 2006, Dr. Yamanaka of Kyoto University was the first to make pluripotent stem cells from fully differentiated adult cells. These cells, called induced pluripotent stem cells (iPSCs), share many qualities with embryonic stem cells. iPSCs are reprogrammed to become pluripotent by exposure to factors that control the expression of genes within the adult cell. These four genes, Oct4, Sox2, cMyc, and Klf4, are collectively referred to as Yamanaka factors.
Click here for more information. |
|
Erythropoietin guides multipotent hematopoietic progenitor cells toward an
erythroid fate.
Hematopoietic stem cells (HSCs) are found in the
bone marrow and give rise to myeloid and lymphoid blood cells through a process
of differentiation. Red blood cells, a.k.a. erythrocytes, come from the myeloid
progenitor cells. Progenitor cells are cells that differentiate into other types
of cells. Erythropoietin (Epo) is an erythroid stress cytokine, which helps in
the development of committed erythroid progenitors. During physiological
emergencies, such as anemia, the levels of erythropoietin can increase in order
to elevate the production of certain cell types. The researchers of this
experiment demonstrate the ability of Epo to suppress the differentiation of
HSCs into any cells other than erythroid cells.
Click here for more information. |
|
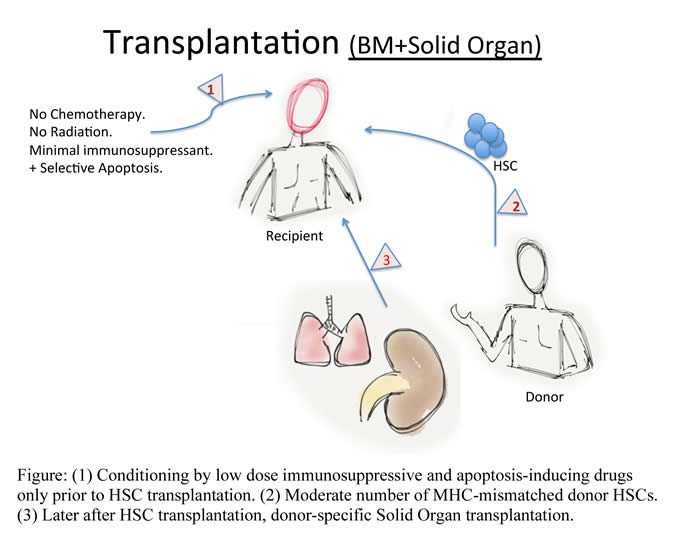
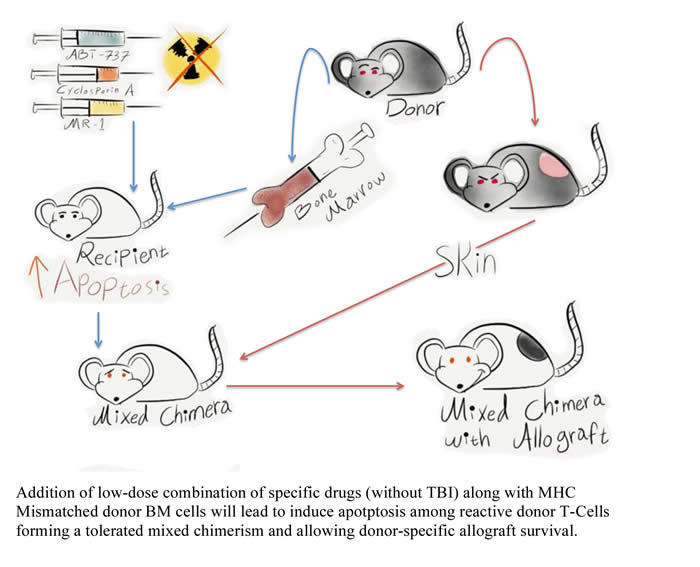
Targeting apoptosis to induce mixed hematopoietic chimerism and long-term allograft survival without myelosupressive conditioning in mice.
In the era of modern medicine, the goal
of transplantation is to achieve effective therapies with minimal use of toxic
drugs. This will enhance prognosis and lifestyle of patients by evading the
current use of drugs. Bone marrow transplantation (BMT) has been used
therapeutically for the treatment of non-solid cancers (leukemia and lymphoma)
but also; there is a recent trend to use BMT to aid the transplantation of a
solid organ. The organ will be from the same donor as the bone marrow cells.
This will reduced the use of toxic drugs and lower immune rejection as well as
suppress bone marrow function (Myelosupression).
Click here for more information. |
|
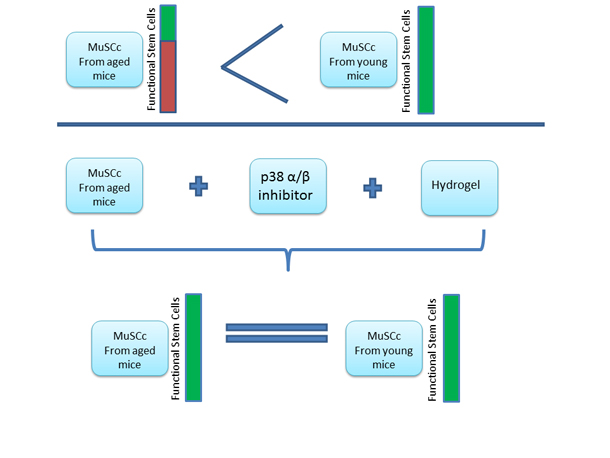
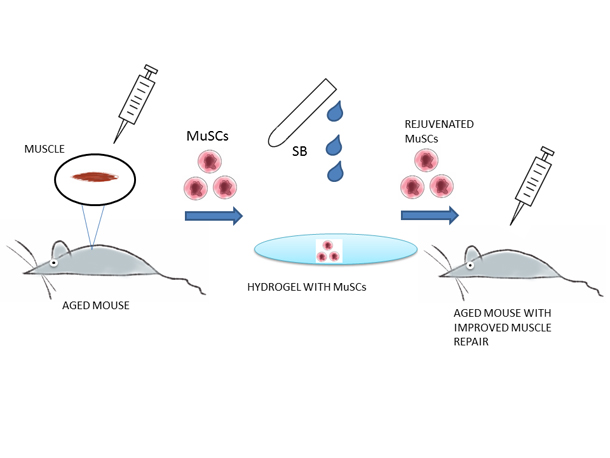
Rejuvenation of the muscle stem cell population restores strength to injured aged muscles.
Our muscles have the natural capability to regenerate when damaged. The main cells involved in the regeneration are the Satellite Cells within the skeletal muscles (one’s that help you move your arms, legs etc.). As one age, the body’s ability to regenerate and repair damaged muscles diminishes. This has been attributed to the satellite cells (also known as Skeletal Muscle Stem Cells) losing their “vigor”, simply put they are not as effective as their young counterparts.
Click here for more information. |
|
Thalidomide corrects impaired mesenchymal stem cell function in inducing tolerogenic DCs in patients with immune thrombocytopenia
Multiple myeloma is an incurable condition in which genetically mutated plasma cells form tumors in bone and disrupt blood and platelet formation. These mutated plasma cells also release antibodies that cause organ failure. Multiple myeloma can be managed with chemotherapy and marrow replacement, however in rare cases these forms of treatment can lead to the development of an autoimmune disorder called immune thrombocytopenia (ITP). Patients with ITP produce antibodies that attack platelets and impair the patient’s ability to clot, thus causing excessive bleeding. These patients may also experience impaired activity and functionality of their mesenchymal stem cells (MSC), which are stem cells that have the potential to suppress the immune system. In this study, researchers investigated whether Thalidomide, a drug that alters the immune system, can correct MSC impairment in patients with ITP.
Click here for more information. |
|
mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation.
Mesenchymal stem cells (MSCs) are one type of stem cell found primarily in the adult bone marrow and adipose tissue. MSCs can form all types of cells such as bone, muscle, tendon, fat, neuron and kidney cells. MSCs can home to sites of inflammation. At the site of inflammation, MSCs exhibit suppress the immune functions. This means that once MSCs target inflammation, they are very effective at decreasing inflammation. However, researchers have run into some problems when studying MSCs for immunosuppression, including, 1) MSCs do not always home very effectively to the region of inflammation; and 2) The immunomodulatory properties of MSCs are highly variable, depending on the environment (in vitro, or outside of the body, vs. in vivo, or inside of the body). This study focused on engineering MSCs, via a process called mRNA transfection, with three specific properties in order to combat both problems: 1) increasing the ability of MSCs to home to inflammation; and 2) adding a specific immunosuppressive effect to the MSCs.
Click here for additional information. |
|
Derivation of naïve human embryonic stem cells.
At first glance, the topic of human embryonic stem cells (hESCs) may spark a wide range of controversy. The cryopreserved human embryos used in this paper were donated from fertility clinic patients. Thus, these human embryos would have been destroyed regardless of this experiment. The author of this paper used two new routes to generate “naïve” hESCs, which represent the developmentally earliest state described for human established cells. First, existing hESC lines in a later “primed” state can be reversed to a “naïve” state by pre-exposure to a “naïve” culture. Second, a new line of hESCs was established using a frozen human embryo under “naïve” culture conditions. It is important to note that these two routes involve the acquisition of embryonic cells from in vitro fertilization. These human “naïve” cells then met the mouse criteria for the “naïve” state. There are practical advantages for these human “naïve” cells, which may include an increase in the developmental potential and a more accurate correlation to the mouse embryonic stem cell data.
Click here for additional information. |
|
Numb-deficient satellite cells have regeneration and proliferation defects.
Comprehension of the molecular mechanisms regulating the regenerative and proliferative capacity of muscle stem cells may hold potential in developing therapeutic approaches to regenerate injured or atrophied muscle.
Click here for additional information. |
|
Reprogramming in vivo Produces Teratomas and iPS Cells with Totipotency Features.
Mature cells are very different from each other, and can be classified by their ‘lineage’. This lineage, or line of cells from which one cell comes from, share many characteristics with each other, and generally travel from progenitor to fully differentiated cell in the body (in vivo). However, under certain conditions, a fully differentiated cell can revert back to become a progenitor cell. If done completely, a cell type can revert all the way back to its original cell type, a stem cell. These cells give rise to the different lineages of an organism, such as blood (hematopoietic) cells, organ lining (epithelial) cells, stomach cells, brain cells, skin cells, etc. This is important because if a cell population in your heart is damaged and unable to repair itself, it is possible to take another cell type, revert it back to its ‘stem cell’ state, and then differentiate into heart cells to help with recovery. While this process is well studied as a lab technique, in vitro, several studies have indicated that this phenomenon might be able to occur in a living organism, in vivo, under the right circumstances and induced chemically.
Click here for additional information. |
|
Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells.
The Notch pathway or signaling is a system cells use to control growth and development from an unspecialized cell state to a more specialized form that performs a unique activity. It consists of several receptors such as Notch1 and Notch2 present on the cell surface that bind specifically to certain proteins namely, Jagged 1 (Jag1) and Delta1 (Dll1). Upon binding, signals are transmitted into the cell and depending on the type of signal the cell changes accordingly. It has been shown that this pathway is involved in the development of blood from precursor cells, referred to as hematopoiesis, and endothelial cells in both vertebrates and invertebrates. In this paper, the investigators demonstrate that Notch signaling drive the formation of blood cells while preventing endothelial cell formation of human precursor cells that can become either type.
Click here for additional information. |
|
Lowest numbers of primary CD8+ T cells can reconstitute protective immunity upon adoptive immunotherapy.
Patients inflicted with certain cancers of the blood or bone marrow, such as multiple myeloma or leukemia, are often unable to effectively replenish the body’s demand for hematopoietic stem cells. These cells are primarily responsible for the constant renewal of blood, which provides constant maintenance and immune protection of the human body, and the production of various specialized cells, like macrophages, platelets, and T-cells. To restore these stem cells, hematopoietic stem cell transplants have become useful treatment options. Unlike autologous transplants, in which patients receive their own stem cells, allogeneic transplants involve the transfer of stem cells from an individual donor (usually a sibling).
Click here for additional information. |
|
Stem cells act through multiple mechanisms to benefit mice with
neurodegenerative metabolic disease.
Neural stem cells (NSCs) have a
range of therapeutic actions in neurodegenerative diseases. This study
investigated the benefit of neural stem cells in a mouse model of Sandhoff
disease, which results from a deletion of the b-chain in b-hexosaminidase (Hex),
causing deficiencies in the isoenzymes HexA and HexB leading to accumulation of
gangliosides within lysosomes throughout the central nervous system. Children
with this disease have severe mental retardation and motor dysfunction and death
usually occurs in infancy.
Click
here for additional information. |
|
A Novel Function of Interleukin-10 Promoting Self-Renewal of
Hematopoietic Stem Cells.
There is evidence that Hematopoietic Stem
Cells (HSCs) exhibit the unique ability to self-renew. However, little is known
about the mechanisms associated with promoting this ability during the
asymmetric division of HSCs. This paper examines the role of an immune
regulator known as Interleukin-10 (IL-10) on HSC self-renewal using animal
models divided into two groups that differed in genotype. The genotypic
difference in the two groups was used in order to identify the origin of the
cells being observed as either being derived from the donor or from the
recipient (Kang et al, 2007). Also used in this study was an animal model that
lacks the gene for IL-10, known as IL-10 knockout (KO) mice.
Click here for additional information. |
|
Canine Embryo-Derived Stem Cells – Towards Clinical Relevant
Animal Models for Evaluating Efficacy and Safety of Cell therapies.
Embryonic stem cells (ESCs) can generate all types of cells,
which could be used to replace damaged cells or to regenerate damaged tissues.
ESCs are of special interest because of their potential clinical application in
treating diseases, and replacing pathological cells and tissues. Currently,
ESCs have been extensively studied in mouse; however, understanding the
properties of these cells in other animal models is necessary, in particular the
animal close to human is need for future development of technologies for humans.
Canine (dog) ESCs also have some advantages over mouse ESCs. Canines have a
longer life-span than mice; this will enable scientists to do long-term studies
with ESCs that are currently impossible in mouse models. Canine diseases are
similar to human diseases; this similarity makes them a better model for human
diseases. The significant size difference between canine and mouse will enable
scientist to study and anticipate potential problems when stem cell therapies
are scaled up for human delivery.
Read
more. |
|
Functions as a Regulator of Human Hematopoietic Stem Cell or
Progenitor Cells.
This summary relates to the role of the Nephroblastoma gene
(NOV CCN3) on the functions of Hematopoietic Stem Cells (HSCs) and
their progenitors. HSCs are present in the adult bone marrow close to the
endosteum where the oxygen levels are low. HSCs can self-renew, which provide
them with the ability to maintain their total numbers. HSCs demonstrate
pluripotency which allows them to differentiate into specialized cells of the
immune and blood systems. Hematopoiesis is the process by which blood cells are
generated from HSCs. In contrast to HSCs, the progenitors are committed cells
and have therefore lost their stemness or pluripotent properties. Due to their
limited ability to form multiple lineages, they are referred as multipotent
cells.
Read more. |
|
Erythropoietin in Cancer: Presumption of Innocence?
One of the major obstacles encountered by drug manufactures developing cancer
therapies is the inability to specifically target cancer cells without effecting
normal cellular function. Most drugs currently in use exert their effects by
inhibiting various pathways crucial to cell cycling and growth. In this way,
these drugs inhibit tumor growth. The cancer drugs affect normal tissues that
rely on continuous cellular renewal, which is the basis for major side effects.
These include hair, gut and bone marrow progenitors also inhibited as a
side-effect. These inhibitory side effects give rise to the well recognized
presentation of hair loss, anemia, and immunosuppression in a patient undergoing
chemotherapy.
Read more. |
|
The Future (R) evolution of Preimplantation Genetic
Diagnosis/Human Leukocyte Antigen Testing: Ethical Reflections.
Preimplantation genetic diagnosis (PGD) with HLA (human leukocyte antigen)
testing has increasingly become a topic for study as it may prove useful in
identifying potentially life-saving properties of umbilical cord blood cells.
The ethical issues in conjunction with this type of testing abound, as parents
have more reason to conceive another child in order to treat diseased offspring.
The method by which parents choose to attain the ends of treating an ill child
can vary with regards to PGD/HLA testing, as will be illustrated further in this
review.
Read more. |
|
Isolation, characterization, and in vitro and in vivo
differentiation of putative thecal stem cells.
Thecal cells are specialized cells that make up a layer of the ovarian
follicles. Their primary purpose is the production of a hormone that is the
precursor to estrogen and structural support which are performed by the theca
interna and theca externa cells, respectively. Originally the primary goal of
the authors was to isolate female germ line stem cells. Germ cells are those
cells that contain half the DNA necessary for reproduction. They come from
either a male or female and produce the egg and sperm of an organism. The
discovery is that the apparent stem cells were instead, somatic cells rather
than germ cells. Thus, the authors proceeded to characterize the isolated
somatic cells as well as functional studies.
Read
More. |
|
Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow
Cell Transplantation and Bone Marrow Stimulation with Granulocyte
Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial.
Spinal Cord injury (SCI) patients presently have no curative form of
treatments. The lack of treatments is mainly due to the seeming inability of
the Spinal Cord to regenerate after trauma. Previous experiments have deduced
the possibility of Bone Marrow Cell transplantation as a prospect for
therapeutic intervention after SCI. The use of bone marrow harvested from the
same patient shows a glimpse of hope since these cells have been shown to revive
some of the cells which could in turn stimulate the re-growth of the spinal
cord. This re-growth could lead to functional improvement, although the
progress may vary without a cure.
Read More. |
|
BM-MSC Promote Neuronal Networks with Functional Synaptic
Transmission After Transplantation into Mice with
Neurodegeneration.
Bone marrow derived mesenchymal stem cells (BM-MSCs) are stem cells found in
the bone marrow and have the potential of differentiation into various cell
types provided with optimal conditions. Differentiation is a process by which
stem cells are naturally or experimentally transformed into other cell types
that possess specialized function such as neural cells that are found in the
nervous system. It has been hypothesized that MSCs cells would be able to
differentiate and give rise to neural cells within the brain. Researchers
believe that by transplanting these cells into damaged tissues may act as an
innovative treatment by reversing the damage. One such neurodegenerative
disorder is Niemann-Pick Disease type C (NP-C). NP is an inherited metabolic
disorder characterized by accumulation of lipids in the brain due to a defect in
cholesterol transport between brain cells.
Read
More. |
|
Selection of Embryonic Stem Cell-Derived Enhanced Green
Fluorescent Protein-Positive Dopamine Neurons Using the Tyrosine
HydroxylasePromoter Is Confounded by Reporter Gene Expression in Immature Cell
Populations.
Embryonic stem cells (ESCs) have been proposed for the treatment of
Parkinson’s Disease (PD) as well as experimental models. Despite their
potential, there are scientific problems with ESCs. They are difficult to
control mainly to their ease to commit to cells of three germ layers. This
uncontrolled growth often leads to unwanted tumor or teratoma formation. The
researchers of this article reasoned that using a mixed population of ESCs would
more likely result in teratoma formation than a purified population. Although
fewer dopamine neurons are acquired from a purified population, the researchers
argued that teratomas overshadow any behavioral improvement in the PD model.
For this reason, they attempted to eradicate the possibility of teratoma
formation by removing the undesired proliferating cells and select for an ideal
cell population to derive dopamine neurons. The selected neurons are intended
for transplantation into animal model of PD.
Read
More. |
|
Cardiac stem cells in brown adipose tissue express CD133 and
induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes.
Researchers have been working on a way to repair damaged cardiac
tissue after a heart attack. Altering genes and transplanting stem cells into
the affected area are two methods that show the most promise of working. This
paper focuses on a group of newborn fat cells which could be a source of cardiac
cells that could be used for the repair process. The authors also see whether
mesenchymal (bone) stem cells when compared with the hematopoietic (blood and
immune) stem cells are better at becoming these cardiac cells.
Read More
|
|
