(Back)
George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE, Rawls A, Rando TA, Wilson-Rawls J. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci U S A. 2013; 110(46):18549-18554
Summarized by: Justin R. Lucas, Fall 2014
Lay Summary
Comprehension of the molecular mechanisms regulating the regenerative and proliferative capacity of muscle stem cells may hold potential in developing therapeutic approaches to regenerate injured or atrophied muscle.
Stem cells are long-lasting, primitive cells that have the capability to divide and make both cells that will go on to mature and develop specialized functions (i.e., differentiate) as well as cells that are exact copies of the original parent stem cell (i.e., proliferate). Stem cells are able to maintain a long life-span by remaining in a state of relative inactivity, or quiescence. During injury or stress, stem cells become activated and divide to meet the needs of the stress, such as in replacing the skin lost from a cut or an abrasion. Satellite cells are a population of stem cells found in muscle fibers that allow for muscle growth and repair following injury.
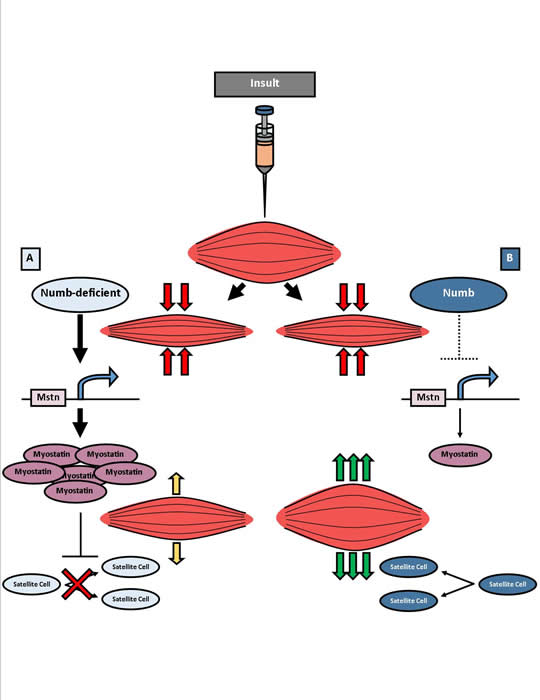
The purpose of the study was to examine the role of Numb in regulating satellite cell activation, proliferation, and differentiation. Numb is a gene whose expression has been shown to influence satellite cells’ decision to either differentiate or proliferate. Based on the current model of Numb function, Numb influences this decision through its ability to disrupt the signaling pathway of Notch. Notch is a receptor whose stimulation promotes cells to remain in a primitive, quiescent state and retain their proliferative capacity instead of differentiating into the mature cells that participate in muscle repair. This model of cell fate control cites Numb’s ability to inhibit the Notch signaling pathway, which occurs through the targeted degradation of the Notch receptor, as the method by which Numb regulates cell fate decisions.
This study presents a new model, or facet, of Numb’s function in muscle regeneration. According to the findings presented in this study, Numb’s regulation of satellite cell fate is not through the inhibition of Notch signaling but rather through control of the expression of myostatin, a cellular growth factor that regulates the size of muscle cells by mediating the balance between proliferation and differentiation of muscle stem cells.
The authors used several agents to injure muscle cells. After this, studies were conducted to understand the role of Numb in satellite cell activation, proliferation, and differentiation. Thje studies used deletion of Numb in mice; the selective deletion of Numb in adult mice; the overexpression of Numb and, the purposeful decrease in myostatin expression.
The results showed that when Numb was knocked out in adult mice, muscle repair was decreased. The loss of Numb resulted in an overall decrease in the number of satellite cells per muscle fiber. Finally, the results showed that Numb primarily regulates satellite cell proliferation via control of myostatin levels. This finding was different from what is known about numb as an inhibitor of notch. This was confirmed by increasing Numb to decrease myostatin expression but not the gene products generated through notch signaling. The results of this study show that, contrary to the current model, Numb regulates muscle stem cell proliferation through control of myostatin expression, thereby affecting muscle cell repair.
Scientific Summary
A better understanding of the molecular pathways that are involved in the response of satellite cells following injury to muscle could lead to the development of treatments for muscle loss and atrophy among other muscle-related diseases.
The discussed study examined the role of Numb in the regulation of satellite cell activation, proliferation, and differentiation. Numb expression has been shown to influence the satellite cell binary fate decision to either differentiate or proliferate (George, et al., 2013). Currently, studies indicated that Numb can inhibit the notch signaling pathway via polyubiquitination and subsequent degradation to control cell fate. This study indicated that Numb has a regulatory role in myostatin expression, which affected satellite and progenitor muscle cell proliferation and differentiation (George, et al., 2013).
In order to show that Numb plays a role in muscle repair, muscle from the tibialis anterior (TA) was injured in Numb-positive and Numb-deficient mice. This was achieved by the use of cre-lox recombination system in which Numb and Numblike were targeted and excised from murine satellite cells. TA muscles were injured via direct injection of BaCl2 into the TA. Muscle fibers were tested for Pax7 expression, which is associated with cycling quiescence. Pax 7 identified the satellite cells. The results showed that Numb-Numb-like-deficient mouse TA muscles had a smaller cross sectional area than control mice post injury, indicating that Numb may play a role in muscle growth and repair. Targeted excision of Numb in adult mice indicated that the deficiency in muscle repair in the knockout mice was not due to a deficit of myogenic progenitor cells (MPCs) during development, which could occur by premature Numb excision. Conditional knockout of Numb and Numb-like alleles in mice at a controlled time point in the adult progeny mirrored the outcome of the other experiments, supporting a role for Numb in muscle repair.
The study next examined the effect of Numb on satellite cell in post-injury. Cell quiescence, proliferation and satellite cell markers were indicated Pax7, Ki67 and Syn4, respectively. The results showed that the proportion of quiescent Pax7+ve satellite cells to proliferating Ki67+ve cells remained constant; however, there was an overall decrease in satellite cell number per muscle fiber as evidenced by the decrease in Pax7+ve cell count. Evaluation of caspase 3/7 activity indicated that the satellite cell deficit in Numb-deficient myofibers was not due to apoptosis. The authors therefor studied notch activity in Numb-deficient mice. The loss of Numb did not cause a significant change in Notch. However, there was a significant increase in myostatin and p21, indicating that Notch was not the primary target of Numb in its role as a mediator of satellite cell fate decisions (George, et al., 2013).
To verify the effect of Numb on myostatin’s effect on muscle stem cell proliferation and differentiation, Numb was overexpressed in primary myocytes, and myostatin was knocked down with specific-siRNA. The results indicated that Numb regulated myostatin expression in injury. Overall, contrary to the current model, Numb regulates muscle stem cell proliferation through control of myostatin expression, thereby affecting muscle cell repair.
Citations:
George RM et al, Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci USA 2013;110: 18549-54.
Le Grant F et al, Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2008;19:628-33.
(Back) |
