 Nikhat Parveen, Ph.D. Nikhat Parveen, Ph.D.
Associate Professor
Office: ICPH-E350T
Tel: 973-972-5218
Lab: ICPH-E-310N.1
Tel: 973-972-4437
NJMS Faculty Profile |
Virulence factors of Pseudomonas aeruginosa and Lyme disease spirochete, Borrelia burgdorferi
My laboratory is studying the molecular basis of pathogenesis of bacterial species, Borrelia burgdorferi, Treponema pallidum and Pseudomonas aeruginosa. These clinically important bacterial pathogens are transmitted to humans using different mechanisms and also show different disease manifestations. B. burgdorferi is transmitted by Ixodes tick vector, T. pallidum by sexual contact and P. aeruginosa, a ubiquitously present organism, is transmitted through ventilation or by direct contact of the patient with the contaminated source.
B. burgdorferi, a spirochete, is causative agent of Lyme disease, a multisystemic illness that affects various organs including joints, heart, nervous system and skin. If untreated, 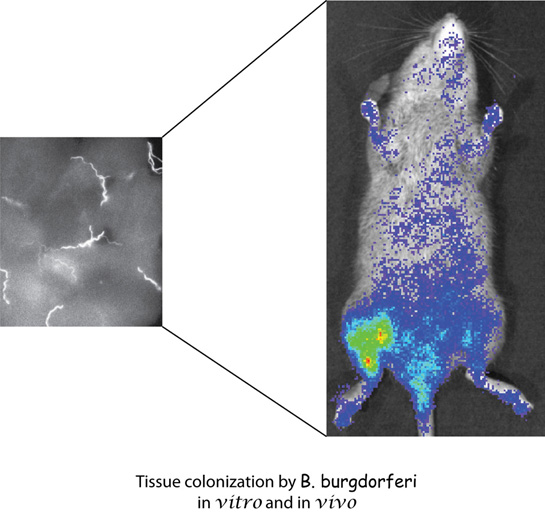 it may result in chronic disease with the symptoms including arthritis, acrodermatitis or neuroborreliosis. It is an extracellular pathogen often found adhering to the host cells in the biopsy specimens of the patients. We have been studying the molecular mechanisms involved in the attachment of Lyme disease spirochetes to a variety of host cells. The specific interaction between the spirochete and host cells may be responsible for the tissue tropism exhibited by B. burgdorferi. Our objective is to understand whether different B.burgdorferi adhesins show affinity for different host receptors on various host cells. We use genetics, biochemical techniques and tissue culture system to identify and characterize the bacterial and host molecules involved in this interaction in vitro. We have already identified two types of glycosaminoglycan receptors on mammalian cells that are recognized by several B. burgdorferi proteins and we are further characterizing this interaction. Mouse is a natural host of B. burgdorferi and C3H mice show several manifestations of Lyme disease observed in humans. We have recently adapted firefly luciferase-based detection system for B. burgdorferi. Using a combination of bioluminescent B. burgdorferi and mouse model of infection, we will further analyze the contribution of each bacterial ligand-host receptor interaction in Lyme pathogenesis. Tissue colonization by the spirochetes will be monitored non-invasively by employing in vivo imaging system. Recently, we have initiated studies to understand molecular basis of T. pallidum pathogenesis using this as a surrogate system.
it may result in chronic disease with the symptoms including arthritis, acrodermatitis or neuroborreliosis. It is an extracellular pathogen often found adhering to the host cells in the biopsy specimens of the patients. We have been studying the molecular mechanisms involved in the attachment of Lyme disease spirochetes to a variety of host cells. The specific interaction between the spirochete and host cells may be responsible for the tissue tropism exhibited by B. burgdorferi. Our objective is to understand whether different B.burgdorferi adhesins show affinity for different host receptors on various host cells. We use genetics, biochemical techniques and tissue culture system to identify and characterize the bacterial and host molecules involved in this interaction in vitro. We have already identified two types of glycosaminoglycan receptors on mammalian cells that are recognized by several B. burgdorferi proteins and we are further characterizing this interaction. Mouse is a natural host of B. burgdorferi and C3H mice show several manifestations of Lyme disease observed in humans. We have recently adapted firefly luciferase-based detection system for B. burgdorferi. Using a combination of bioluminescent B. burgdorferi and mouse model of infection, we will further analyze the contribution of each bacterial ligand-host receptor interaction in Lyme pathogenesis. Tissue colonization by the spirochetes will be monitored non-invasively by employing in vivo imaging system. Recently, we have initiated studies to understand molecular basis of T. pallidum pathogenesis using this as a surrogate system.
P. aeruginosa is an opportunistic pathogen and produces a wide variety of virulence factors. 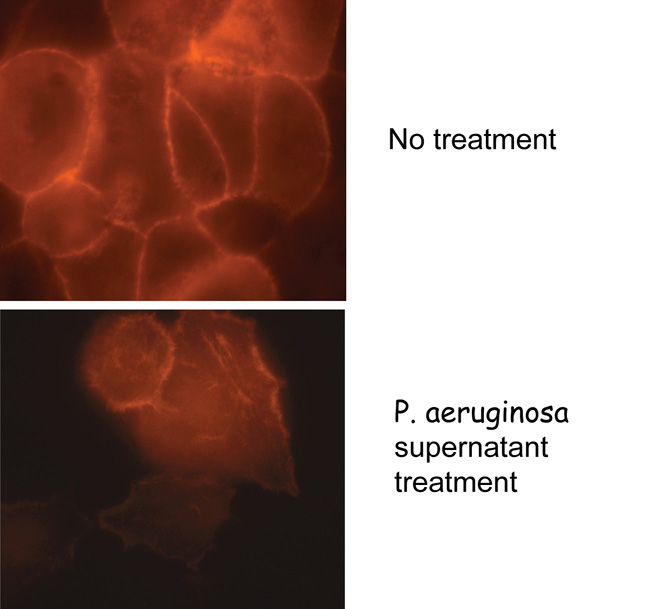 It results in a variety of illnesses and is responsible for high morbidity and mortality in immunocompromised and elderly patients. Due to a highly adaptable nature of P. aeruginosa and its ability to survive even in detergents, it is a major contributor to infections in the hospital environment. We have been studying the quorum-sensing mediated induction of several virulence factors in this organism both as free-living organism and in association with its different hosts. We will assess the role of selected virulence factors in biofilm formation while P. aeruginosa is present in communities along with the other organisms. Our current focus is to investigate genetics of production and regulation of PrpL protease and pyocyanin pigment of P. aeruginosa and examine the roles of these virulence factors in tissue destruction. The roles of these two virulence factors in corneal damage, in burn wounds and in the cystic fibrosis patients will then be examined.
It results in a variety of illnesses and is responsible for high morbidity and mortality in immunocompromised and elderly patients. Due to a highly adaptable nature of P. aeruginosa and its ability to survive even in detergents, it is a major contributor to infections in the hospital environment. We have been studying the quorum-sensing mediated induction of several virulence factors in this organism both as free-living organism and in association with its different hosts. We will assess the role of selected virulence factors in biofilm formation while P. aeruginosa is present in communities along with the other organisms. Our current focus is to investigate genetics of production and regulation of PrpL protease and pyocyanin pigment of P. aeruginosa and examine the roles of these virulence factors in tissue destruction. The roles of these two virulence factors in corneal damage, in burn wounds and in the cystic fibrosis patients will then be examined.
Selected Publications
-
Djokic, V., Giacani, L., and N. Parveen. 2019. Analysis of host cell binding specificity mediated by the Tp0136 adhesin of the syphilis agent Treponema pallidum subsp. pallidum. 9;13(5):e0007401. doi: 10.1371/journal.pntd.0007401.
- Parveen, N., Fernandez, M.C., Haynes, A.M., Zhang, R.L., Godornes, B.C., Centurion-Lara ,A., Giacani L. 2019. Non-pathogenic Borrelia burgdorferi expressing Treponema pallidum TprK and Tp0435 antigens as a novel approach to evaluate syphilis vaccine candidates. Feb 20. pii: S0264-410X(19)30217-8. doi: 10.1016/j.vaccine.2019.02.022
- Djokic, V., Primus, S., Akoolo, L., Chakraborti, M. and N. Parveen. 2018. Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affect diseases severity. Frontiers in Immunology. 7;9:2891. doi: 10.3389/fimmu.2018.02891
- Bhanot, P., and N. Parveen. 2018. Severity of symptoms of babesiosis alone or with Lyme disease in the mouse infection model. Invited review. International Journal of Parasitology. International Journal of Parasitology. 49(2):145-151. doi: 10.1016/j.ijpara.2018.06.006.
- Primus, S., Akoolo, L., Schlachter, S., N. Parveen. 2018. Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS One. 10;13(5):e0196748. doi: 10.1371/journal.pone.0196748.
- Djokic, V., Akoolo, L., and N. Parveen. 2018. Babesia microti Infection Changes Host Spleen Architecture and Is Cleared by a Th1 Immune Response. Frontiers in Microbiology. 9:85. doi: 10.3389/fmicb.2018.00085. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5797759
- Primus, S., Akoolo, L., Schlachter, S., N. Parveen. 2018. Screening of patient blood samples for babesiosis using enzymatic assays. Tick and Tick Borne Diseases. 9(2):302-306. doi:10.1016/j.ttbdis.2017.11.003
- Schlachter, S., Seshu, J., Lin, T., Norris, S., N. Parveen. 2018. Borrelia burgdorferi glycosaminoglycan binding protein, Bgp in the B31 strain is not essential for infectivity despite facilitating adherence and tissue colonization. Infection and Immunity. 22;86(2). pii: e00667-17. doi: 10.1128/IAI.00667-17
- Akoolo, L., Schlachter, S., Khan, K., Alter, L., Rojtman , A. D., Gedroic, K., Bhanot, P., and N. Parveen. 2017. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiology 17:16-24. doi:10.1186/s12866-017-0929-2
- Chan, K., Nasereddin, T., Alter, L., Centurion-Lara, A., Giacani,G., and N. Parveen. 2016. “Treponema pallidum Lipoprotein TP0435 Expressed in Borrelia burgdorferi Produces Multiple Surface/Periplasmic Isoforms and mediates Adherence”. Nature Scientific Reports 6:25593. doi:10.1038/srep25593.
Patent
"Diagnostic assay for Lyme disease and other tick-borne diseases"
Provisional Application No. 61/877,479
Ref. No. 11-83
International Patent (PCT) provisional filed
Recent Grants
- "Placental colonization by Treponema pallidum, congenital syphilis & novel vaccine approach"
NIH U01 (Contact PI) $2,947,538 8/15-1/20
(With Cayetano University at Peru and University of Washington, Seattle, USA)
- "Borrelia burgdorferi-glycosaminoglycan interactions and Lyme disease pathogenesis"
NIH R01 $1,707,600 7/11-6/16
(Received 9 percentile score)
- "Sensitive and specific detection of Lyme disease bacteria by real-time PCR"
NJ Health Foundation $70,000 1/15-12/15
- "Detection of Lyme spirochetes in patients by real-time PCR"
OTC, Rutgers Translational Mini-grant $10,000 4/15-3/16
- "A unique approach to identify markers for congenital syphilis and neurosyphilis"
NIH Challenge grant $367,600 9/10-8/12
Award
Excellence in Research Award 2015-New Jersey Health Foundation
Training and Positions
1988-1991 Scientist at IARI, New Delhi and Investigator in Indo-US Bilateral Program
1991-1995 Ph.D. in Microbiology, University of Hawaii at Manoa, Honolulu, HI
1996-Nov.2000 Postdoctoral Fellow, mentor: John Leong, Univ. Mass. Med. School, MA
2000-May 2005 Research Assistant Professor, Univ. Mass. Med. School, MA
