Back
Lee J-P, Jeyakumar M, Gonzalez R, Takahashi H, Lee P-J, Baek RC, Clark D,
Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller F-J, Park KI, Butters TD,
Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder
EY. Stem cells act through multiple mechanisms to benefit mice with
neurodegenerative metabolic disease. Nature Med 2007;13:439-47.
Summarized by: Pamela Orellana and Denis Chang, Fall
2007
LAY SUMMARY
Neural stem cells (NSCs) have a range of therapeutic actions in
neurodegenerative diseases. This study investigated the benefit of neural stem
cells in a mouse model of Sandhoff disease, which results from a deletion of the
b-chain in b-hexosaminidase (Hex), causing deficiencies in the isoenzymes HexA
and HexB leading to accumulation of gangliosides within lysosomes throughout the
central nervous system. Children with this disease have severe mental
retardation and motor dysfunction and death usually occurs in infancy.
Four different NSCs were transplanted separately into the forebrain and
cerebellum of newborn Hex deficient mice: 1) mouse NCSs (mNCSs) from a stable,
clonal population of engraftable lacZ-expressing mNSCs from clone C17.2
mice, 2) mNSCs isolated as neurospheres from the telencephalon of E10.5 Rosa 26
mice. 3) human NSCs (hNCSs) isolated from the telencephalic ventricular zone of
late first-trimester human fetal cadavers, 4) hNSCs derived in vitro from human embryonic stem cells. In all four trials, results showed that
symptoms onset was delayed and lifespan was prolonged, an improvement of ~70%
compared to untreated age-matched mice. Motor functions was assessed by rotarod
performance and the righting reflex test and showed that transplanted mice
performed better than untreated mice.
Mechanism of improvement was concluded to be due to NSCs cross correcting
β-hexosaminidase deficiency, which reduces monosialoganglioside (GM2) and
gangliotriaosylceramide (GA2) storage. This was demonstrated using
immunochemistry to assess the distribution and content in the cerebral cortex of
Hex deficient mice transplanted at birth with mouse NSCs. Both GM2 and GA2 were
significantly reduced in the mouse model. The impact of GM2/GA2 storage was also
tested using human NSCs and it showed similar results.
Furthermore, this study suggests that the effectiveness of the cross
corrective enzyme supplied by the NSCs can be improved by reducing the amount of
substrate to be metabolized. In substrate reduction therapy (SRT), imino sugars,
N-butyldeoxynojirimycin (NB-DNJ) or N-butyldeoxygalactonojirimycin (NB-DGJ), are
used to inhibit GM2/GA2 biosynthesis and storage. When transplantation of mouse
NSCs into neonatal cerebrum was combined with SRT administration, the Hex
deficient mice survival was increased by 67-71%.
Transplanted NSCs migrated throughout the Hex deficient mouse, providing the
cross correcting enzyme, and reducing ganglioside storage and inflammation. In
combination with other therapies, it might serve as a better prognosis in
neurodegenerative disorders.
SCIENTIFIC SUMMARY
The goal of
this study was to evaluate the therapeutic potential of neural stem cells (NSCs)
in neuronal disorders, specifically Sandhoff disease (SD), and to assess its
efficiency in collaboration with currently existing strategies.
SD is caused by a deletion of the b-chain in b-hexosaminidase (Hex),
resulting in deficiencies in the isoenzymes HexA (ab) and HexB (bb) and
accumulation of gangliosides within lysosomes throughout the central nervous
system (CNS). Four different NSCs, two mouse NSCs (mNSCs) and two human NSCs
(hNSCs), were transplanted individually into the forebrain and cerebellum of
newborn Hexb-/- SD mice. One population of mNSCs was a stable, clonal
population of engraftable lacZ-expressing mNSCs from clone C17.2 mice
while the other was mNSCs isolated as neurospheres from the telencephalon of
E10.5 Rosa 26 mice. mNSCs from clone C17.2 was selected due to their over
expression of the stemness-like gene myc, which preserved multipotency,
self-renewal, and the undifferentiated state in vitro. “Primary” hNSCs
were isolated from the telencephalic ventricular zone of late first-trimester
human fetal cadavers and maintained in serum-free medium containing bFGF,
heparin, and LIF. “Secondary” hNSCs were derived in vitro from hESCs
and maintained under feeder-free culture conditions. Following transplantation,
a marked improvement was observed in all cases. Improved motor function was
determined by rotarod performance and righting reflex test while the onset of
symptoms was delayed along with prolonged survival (Figure 1).
To investigate the potential mechanism underlying these results, the
distribution of engraftment and the degree to which the replacement of mutant
with wild-type neural cells were studied. Though engraftment in the hindbrain
was less robust, the evident distribution of donor-derived cells in the
forebrain pointed towards neuronal replacement as the mode of action. This,
however, was discredited as being the primary cause following the similarities
that existed in the degree of improvement of motor function and lifespan between
the administration mNSCs prenatally and neonatally, the former resulting in more
cortical neuron production in comparison with the latter.
NSCs were shown to improve motor function and delayed symptom onset by
reconstituting Hex enzyme activity, as they are known to express normal levels
of Hex constitutively, and by reducing microglial activation and macrophage
infiltration, both being hallmarks of SD pathogenesis. The cross-corrective
enzyme decreased levels of glycosphingolipid gangliotriaosylceramide (GA2) and
monosialoganglioside (GM2) storage in SD mice, while the NSCs suppressed levels
of CD11b, F4/80, and CD68, which are markers of microglia/macrophage activation.
Similarly, trials using both primary and secondary hNSCs resulted in marked
improvement in both aspects of motor function and prolonged survival. Results
also suggest similar causes for improvement rather than neuronal replacement
following transplant. In all the experiments involving both the mNSCs and hNSCs,
immunosuppressants were not required, despite the transplants being an allograft
and xenograft, respectively.
Although the findings were only therapeutic, as the mice eventually became
subjected to the disease, this study shows great potential, especially in
cooperation with other treatments. It was shown that when the NSC transplant was
synergized with substrate reduction therapy (SRT), in which imino sugars,
specifically N-butyldeoxynojirimycin or N-butyldeoxygalactonojirimycin, are
introduced orally, it resulted in greater improvements in motor function and
significant delaying of symptom onset as compared to each mechanism utilized
separately. Such strategies, along with others, may be used collaboratively to
provide better prognoses in cases involving SD or other neuronal disorders.
Factors, such as the media in which the NSCs are harvested and cultured, and
the environments under which the NSCs are subject to and grown must be
considered in evaluating the validity of the results obtained in this study. As
stem cells are sensitive to microenvironmental exposure, it is important to bear
in mind the role of the cultures and the sources from which they are derived
prior to transplant. Further studies involving the anti-inflammatory and
immunosuppressive behavior of the NSCs would also be of interest as
immunosuppresants were not required despite the transplants being an allograft
and xenograft.
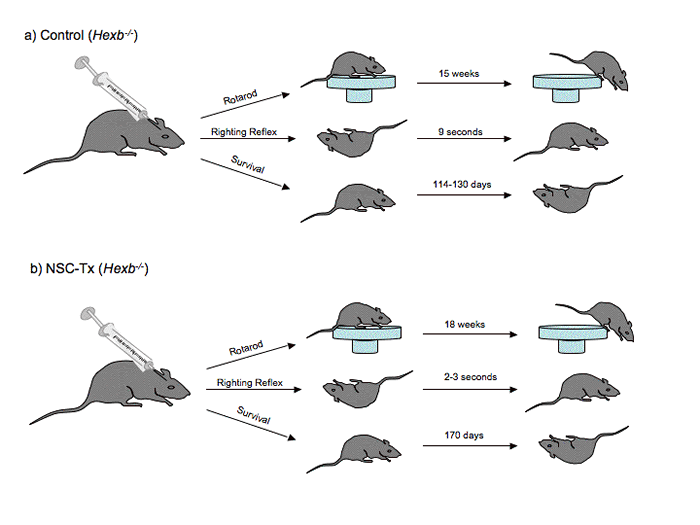
Figure 1
a.) Hexb-/- SD mice transplanted with
fibroblasts, while failed to migrate from the lateral ventricles, exhibited a
gradual decrease in rotarod performance throughout a 15-week period, a righting
reflex test time of 9 seconds and survival for 114-130 days. b.) Hexb-/- SD mice transplanted with NSCs exhibited an increase in motor
function (improved rotarod performance and righting reflex test results) and
prolonged survival (survival time of ~170 days).
Back |
