In The News!
Congratulations!

Dr. Jojo Reyes, a recent PhD graduate from the Center for Immunity & Inflammation was selected as "2024 Outstanding PhD Graduate" by the Rutgers Health New Jersey Medical School Faculty Organization. The award is to recognize academic achievement and contributions to life and missions of New Jersey Medical School. Jojo, who is currently in the Department of Molecular Biology at Princeton University, was notified of this award by New Jersey Medical School Faculty Organization President, Dr. Joshua Kaplan.
Dr. Bhupendra Singh Rawat (Bessman lab) was awarded a 3-year fellowship from the Crohn’s and Colitis Foundation, for his research project titled ‘Hepcidin and iron homeostasis in Inflammatory Bowel Disease.’

Dr. George Yap, Professor, was elected into the esteemed American Academy of Microbiology.
Dr. Yap joins 64 new fellows, who were elected via a highly selective, peer
review process, based on scientific achievements in the advancement of
microbiology.

Dr. Tessa Bergsbaken, Assistant Professor, was selected as the recipient of the 2023 New Jesey Health Foundation Excellence in Research Award for her work on tissue-resident lymphocytes.

Dr. Nicholas Bessman, Assistant Professor and Chancellor Scholar, was being selected to be one of only two Rutgers University nominees for the Searle Scholars Program.


Dr. William Gause and Dr. Patricia Fitzgerald-Bocarsly, were named Fellows of the American Association for the Advancement of Science. Full article in Rutgers Today.

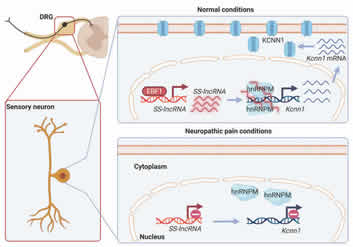
In April 2023, Dr. Yuan-Xiang Tao and his team in Department of Anesthesiology and Center for Immunity and Inflammation published an exciting work in Brain that identified a novel non-coding RNA named sensory neuron-specific lncRNA (SS-lncRNA), for its expression exclusively in the neurons of dorsal root ganglion (DRG) and trigeminal ganglion. They reported that SS-lncRNA relieved neuropathic pain through hnRNPM-mediated KCNN1 rescue. These findings suggest that SS-lncRNA may offer a new therapeutic strategy specific for neuropathic pain.
https://academic.oup.com/brain/advance-article/doi/10.1093/brain/awad110/7100983?searchresult=1
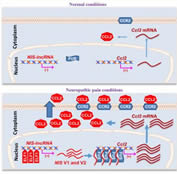
In February 2023, Dr. Yuan-Xiang Tao’s lab published a paper in Br J Anaesth showing that intrathecal NIS-lncRNA antisense oligonucleotides (ASOs) leads to a long-lasting analgesic effect on neuropathic pain caused by nerve trauma, chemotherapy, or diabetes mellitus. This work followed up his team’s previous exciting findings published in J Clin Invest last year (July 2022), which reported that a new identified nerve injury-specific long-noncoding RNA (NIS-lncRNA) promoted neuropathic pain by increasing the expression of CCL2, a small cytokine. Given that ASO strategy is an FDA-approved in clinical treatments of some neurological disorders, NIS-lncRNA ASOs may have potential application in clinical managements of neuropathic pain. A patent related to this work has been applied. This project is funded by a five-year $3.5-million NIH HEAL Initiative grant (2019-2024).
https://www.sciencedirect.com/science/article/pii/S0007091222005682?via%3Dihub
https://www.jci.org/articles/view/153563
Dr. Tessa Bergsbaken, Assistant Professor in Center for Immunity and Inflammation and Department of Pathology, Immunology & Laboratory Medicine, and colleagues published a study in the November 2022 issue of Science Immunology that uses a newly developed mouse model to understand the unique functions of tissue-resident memory T cell subsets. Tissue-resident memory T (Trm) cells are lodged within barrier surfaces and are critical for preventing infection with pathogens that invade these tissues. Dr. Helen Fung, the first author on this paper, found that a small subset of CD103– Trm cells were the primary responders to secondary infection. CD103– Trm cells expanded within the tissue and displayed enhanced TCR-mediated reactivation and cytokine production compared to their CD103+ counterparts. These studies reveal the limited recall potential of CD103+ Trm subsets and the role of CD103– Trm cells as central memory-like T cells within peripheral tissues. Ultimately, these studies suggest that vaccines that lead to the generation of CD103– Trm cells within barrier surfaces could have enhanced efficacy. The findings were highlighted in an article in Rutgers Today.
https://www.science.org/doi/10.1126/sciimmunol.abl9925
In September 2022, Jianya Peng and Chandler Sy from Dr. Mark Siracusa’s lab published a paper in PNAS showing an important role for monocytes in maintaining central nervous system homeostasis following an intestinal parasite challenge. These studies represent an important contribution to the rapidly emerging field of neuroimmunology and suggest that infections occurring at distal sites can dramatically alter the host brain. These findings further showed that a previous infection may make the host less susceptible to subsequent forms of neuroinflammation. Given that neuroinflammation is associated with disorders such as Alzheimer’s and Parkinson’s disease, this work could inform the development of new therapeutic targets to treat neurogenerative conditions.
https://www.pnas.org/doi/10.1073/pnas.2201645119
In August 2022, Darine El-Naccache published a paper in Cell Reports showing an important role for adenosine in triggering type 2 immune responses through binding the Adenosine A2b receptor expressed on intestinal epithelial cells. These studies were part of her Ph.D. dissertation research in Dr. Gause's laboratory, and the studies were done in collaboration with Dr. George Hasko at Columbia University. These findings further showed that the adenosine was derived from extracellular ATP and essentially functions as an endogenous danger signal likely triggered through tissue damage occurring as helminth parasites cross the intestinal barrier. The findings were highlighted in an article in Rutgers Today.
https://www.cell.com/cell-reports/fulltext/S2211-1247(22)00959-7
In August 2022, Dr. Aimee M. Beaulieu, Assistant Professor and Chancellor Scholar, Center for Immunity and Inflammation and Department of Microbiology, Biochemistry, & Molecular Genetics, and colleagues published a study in The Proceedings of the National Academy of Sciences demonstrating that the cytokine, IL-33, is a key regulator of pregnancy progression and type 2 immune responses at the maternal-fetal interface in mice. This collaborative study included researchers from Dr. Beaulieu’s team, and from the teams of Dr. Nataki Douglas in the Center for Immunity and Inflammation and Department of Obstetrics, Gynecology and Reproductive Health at Rutgers NJMS and Dr. Ripla Arora at Michigan State University. Dr. Nuriban Valero-Pacheco, the first author of the paper, demonstrated that pregnant mice lacking the Il33 gene exhibit diverse defects in key physiological and cellular processes in the uterine microenvironment that support pregnancy progression in mice, resulting in impaired fetal and placental development. These defects were associated with diminished Type 2 immune responses by uterine lymphocytes and myeloid cells at the maternal-fetal interface during early pregnancy. Ultimately, this work could inform future efforts to target IL-33 signaling to treat or prevent pregnancy disorders in women.
https://www.pnas.org/doi/10.1073/pnas.2123267119
