Telemeres in Wound Healing
Telomeres in Wound Healing
Senescent cells secrete molecules, a phenomenon called Senescence Associated Secretory Phenotype (SASP). This SASP can be both beneficial and detrimental to the organism as it promotes tissue repair and cancer progression, respectively, albeit by still unknown mechanisms. Molecules secreted from senescent cells rapidly activate a DNA damage response (DDR) and senescence in proliferating cells when added directly to the culture medium. Surprisingly, our data demonstrate that this DDR originates at telomeres and that expression of hTERT can suppress formation of dysfunctional telomeres in response to SASP factors. Our data further reveal that one SASP component, TGF-
β1, also causes telomere dysfunction in normal cells. Since TGF-
β1 plays a critical role in wound healing where it promotes myofibroblast transdifferentiation, we are exploring whether formation of dysfunctional telomeres is required for this process. Indeed, addition of TGF-
β1 to fibroblast cultures triggers telomere dysfunction, induces elevated levels of
α-SMA protein, a signature myofibroblastic gene expression profile, and enhanced contractile ability. In contrast, telomerase-expressing cells are resistant to these changes and to transdifferentiation. Our current studies are characterizing how cytokines, including TGF-
β1 cause the formation of dysfunctional telomeres, and how telomere dysfunction promotes myofibroblast transdifferentiation in humans.
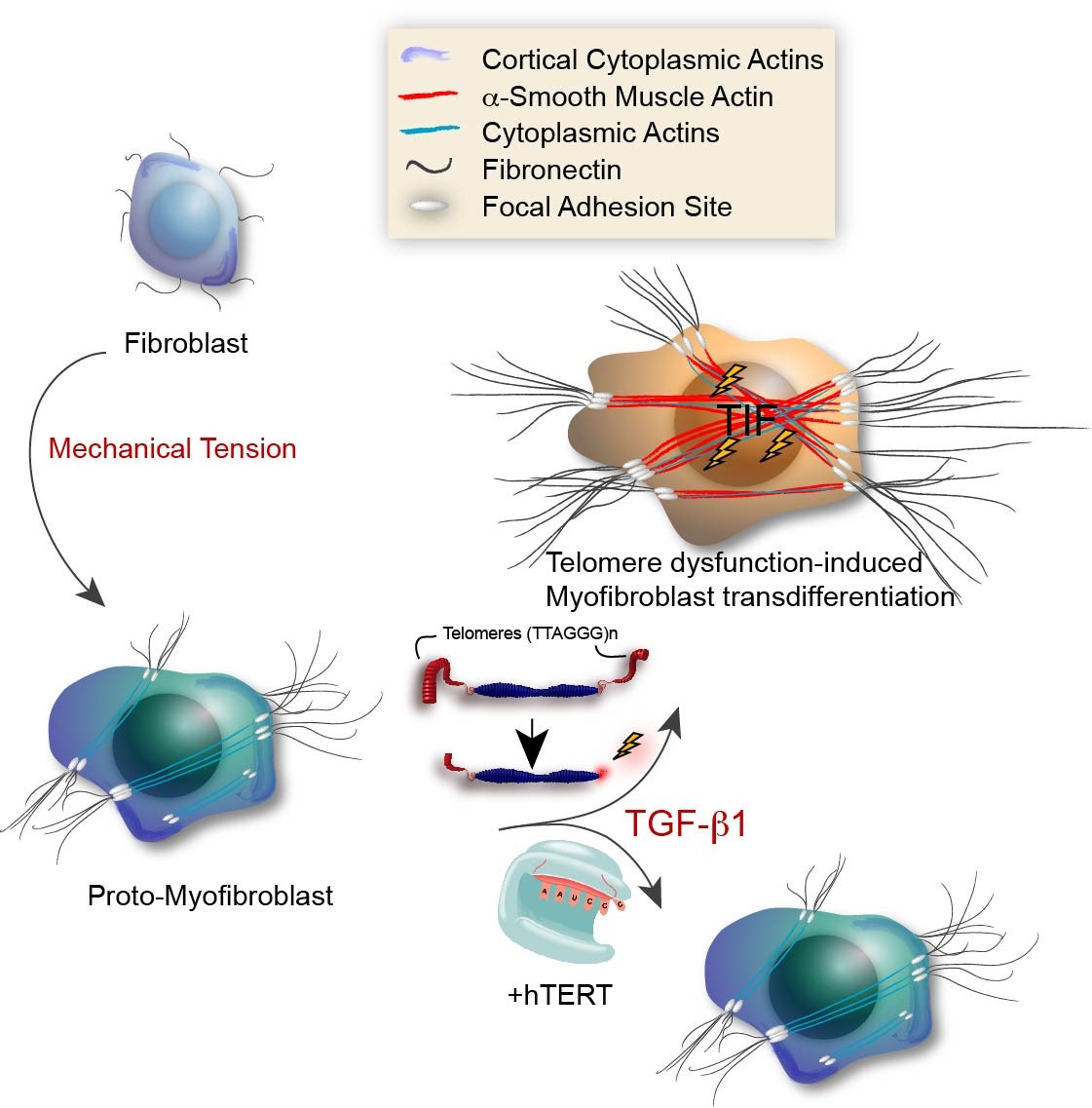
Figure. Model illustrating the role for TDIS in myofibroblast transdifferentiation

