Telomeres in Cancer
Telomeres in Cancer
Testing the hypothesis that replicative senescence evolved as a tumor suppressing mechanism in humans, we demonstrated that the vast majority of cells in hyperplasic regions of three distinct and common pre-malignant human cancers -melanocytic nevi, colonic adenomas, and ductal hyperplasias of the breast, but not cells of their malignant cancer counterparts, displayede hallmarks of TDIS (Suram et al., 2012. The EMBO J). Our data therefore provided evidence that a telomere initiated senescence response suppresses cancer growth in humans (Figure 1).
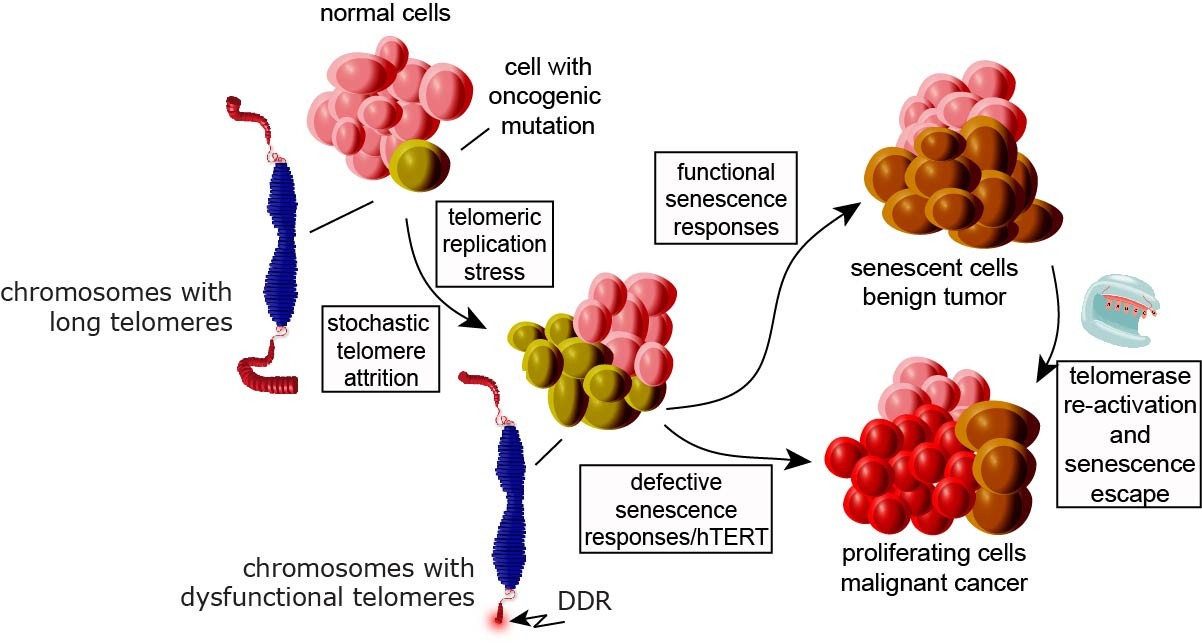 Figure 1: Model illustrating the role of TDIS in suppressing malignant cancer progression in humans
Figure 1: Model illustrating the role of TDIS in suppressing malignant cancer progression in humans
To characterize the causes for TDIS in cancer precursor lesions, we are exploring the possibility that oncogenic signaling, a cancer initiating event, affects telomere length and function. Our data so far revealed that oncogenes, such as H-RasV12 and BRafV600, alter efficiency of telomere replication to such an extent that telomeres erode rapidly, become dysfunctional, and activate cellular senescence (Suram et al., 2012. The EMBO J). Our studies demonstrate that TDIS, accelerated by oncogene-induced DNA replication stress, is a biological response of cells in benign human cancer precursor lesions and suggests that TDIS evolved as a critical tumor suppressing mechanism to protect from malignant cancer progression (Fig. 1).
Although TDIS is now thought to be a critical tumor suppressing mechanism in mammals, whether it truly presents a stable and therefore irreversible barrier to cancer progression is still unclear. Recent results from our laboratory revealed that TDIS, triggered by by expressing HRasG12V, BRafV600E is an unstable barrier that cells can escape following a prolonged period of inactivity. Escape from OIS is due to a combination of events that include derepression of the hTERT promoter and MAP kinase mediated stabilization of c-Myc expression, which together promote re-activation of hTERT expression. Re-activation of telomerase can indeed be detected in cells of human melanocytic skin and breast lesions at early stages during cancer development, suggesting that escape from senescence, facilitated by telomerase reactivation, contributes to cancer progression in humans (Patel et al., 2016, PNAS, in press; Figure 1).

