 Utz Herbig, Ph.D. Utz Herbig, Ph.D.
Associate Professor
Office: Cancer Center F1218
Tel: 973-972-4426
Lab: Cancer Center F Level Bay 10-11
Tel: 973-972-4424
Herbig NJMS Profile
|
Telomeres in Aging, Cancer, and Tissue Repair
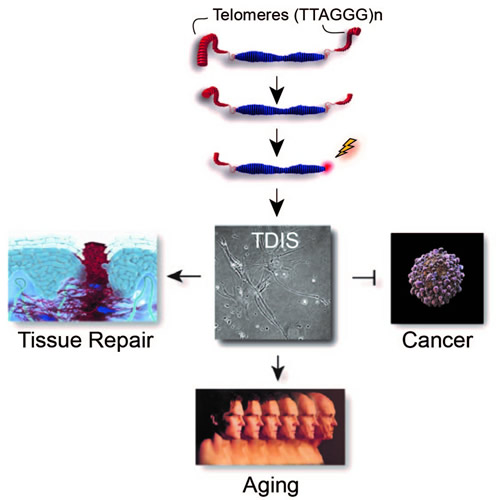
Human somatic cells do not have the ability to proliferate indefinitely. Instead, these cells arrest irreversibly by a process called replicative senescence after undergoing 60-80 cell divisions. Already over 50 years ago, it was speculated that this limited replicative potential served one major purpose: to limit uncontrolled cellular proliferation of cancer cells. It was also proposed that this potential tumor suppressing mechanism would ultimately prevent somatic cells from regenerating our tissues and thereby contribute to aging of humans. These predictions, however, remained untested for decades as the molecular trigger of replicative senescence was unknown. Over the past years, work in our laboratory has revealed that the molecular trigger of replicative senescence is telomere dysfunction (Figure 1), provided evidence that Telomere Dysfunction-Induced Senescence (TDIS) promotes aging, and demonstrated that TDIS indeed suppresses cancer growth in humans. More recently, we discovered another unexpected role for TDIS: that of promoting tissue repair during wound healing. Current research in the laboratory focuses of exploring the causes for telomere erosion, telomere attrition, and telomere dysfunction in addition to characterizing the contributions of TDIS to tumor suppression, aging, and wound healing in humans.
Selected Publications
- Patel, P., Suram, A., Mirani, N., Bischof, O., Herbig, U.2016 Derepression of hTERT Gene Expression Promotes Escape from Oncogene-Induced Cellular Senescence. PNAS; in press
- Boccardi, V., Razdan, N., Kaplunov, J., Mundra, JJ., Kimura, M., Aviv, A., and Herbig, U. 2015. Stn1 is critical for telomere maintenance and long term viability of somatic human cells. Aging Cell 14:372
- Suram A, Herbig U. 2014. The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell. 18. doi: 10.1111
- Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, Fumagalli M, Di Micco R, Mirani N, Gurung RL, Hande MP, d'Adda di Fagagna F, Herbig U. 2012. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. The EMBO Journal. 31: 2839-51
- Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, Herbig U, Longhese MP, d'Adda di Fagagna F. 2012. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nature Cell Biology 14: 355-65
- Benhamed M, Herbig U, Ye T, Dejean A, Bischof O.2012. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nature Cell Biology 14: 266-75
- Jeyapalan, JC., Ferreira, M., Sedivy, JM., and Herbig, U. 2007. Accumulation of Senescent Cells in Mitotic Tissue of Aging Primates. Mechanisms of Ageing and Development. 128:36-44
- Herbig, U., Ferreira, M., Condel, L., Carey, D. and Sedivy, J.M. 2006. Cellular senescence in aging primates. Science 311:1257
Awards and Honors
- Research Scholar Grant Award from American Cancer Society, 2011
- New Scholar in Aging Award; The Ellison Medical Foundation, 2007
- Ruth L. Kirschstein National Research Service Award (NRSA), 2003
- Graduate Student Research Excellence Award in Biological Sciences; Vanderbilt University, 1999
Education
1999 Ph.D. in Molecular Biology, Vanderbilt University, Nashville, TN
1995 Hauptdiplom (Masters of Science) in Chemistry/Biochemistry, Ludwig Maximilians University, Munich, Germany
Positions
2013-present Associate Professor, Microbiology, Biochemistry & Molecular Genetics, Rutgers Biomedical and Health Sciences, Rutgers, The State University of New Jersey
2006-2013 Assistant Professor, Microbiology & Molecular Genetics, University of Medicine and Dentistry of New Jersey (UMDNJ)

