mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation
O. Levy, W. Zhao, L. J. Mortensen, S. LeBlanc, K. Tsang, M. Fu, J. A. Phillips, V. Sagar, P. Anandakumaran, J. Ngai, C. H. Cui, P. Eimon, M. Angel, C. P. Lin, M. F. Yanik, J. M. Karp. Blood 2013; 122 (14): e23
Prepared by: Emily Byrne, 2014
Lay Summary
Mesenchymal stem cells (MSCs) are one type of stem cell found primarily in the adult bone marrow and adipose tissue. MSCs can form all types of cells such as bone, muscle, tendon, fat, neuron and kidney cells. MSCs can home to sites of inflammation. At the site of inflammation, MSCs exhibit suppress the immune functions. This means that once MSCs target inflammation, they are very effective at decreasing inflammation. However, researchers have run into some problems when studying MSCs for immunosuppression, including, 1) MSCs do not always home very effectively to the region of inflammation; and 2) The immunomodulatory properties of MSCs are highly variable, depending on the environment (in vitro, or outside of the body, vs. in vivo, or inside of the body). This study focused on engineering MSCs, via a process called mRNA transfection, with three specific properties in order to combat both problems: 1) increasing the ability of MSCs to home to inflammation; and 2) adding a specific immunosuppressive effect to the MSCs.
In order to increase homing to sites of inflammation, the researchers used DNA transfection to get two adhesion ligands, PSGL-1 and SLeX (which are not found on native MSCs) to the surface of the MSCs. Through a number of experiments, they found that by double transfecting the MSCs with both PSGL-1 and SLeX, the engineered MSCs exhibited an increased ability to roll along inflamed tissue, a process which increases the ease with which MSCs can exit the blood vessel and enter into the site of inflammation. In addition, the ability of MSCs to target or home to points of inflammation was also increased. Rolling and homing were increased both in vitro and in vivo (in mice).
In order to enhance the immunosuppressive ability of MSCs, the researchers used transfection to add a potent immunosuppressing cytokine, IL-10, to the inside of the MSCs. Their hypothesis was that once the MSC homes to a point of inflammation, the IL-10 will be secreted by the MSC and decrease inflammation. Throughout a series of experiments, both in vitro and in vivo, the researchers found that, paired with the addition of the adhesion ligands PSGL-1 and SLeX, the addition of IL-10 to MSCs led to a large decrease in inflammation. Most importantly, the addition of all three factors to the MSCs led to the largest decrease in inflammation, whereas the addition of only one or two of the factors led to a significantly smaller decrease in inflammation.
Through the process cell engineering technique, this study revealed an effective method to engineer MSCs to be effectively decrease inflammation. In a clinical context, this method could be effective in targeted drug delivery.
Scientific Summary
Mesenchymal stem cells (MSCs) can be engineered to express one gene. This study expressed three genes that expressed adhesion ligands (P-selectin glycoprotein ligand-1 (PSGL-1) and Sialyl LewisX (SLeX)) and IL-10. The triple transfection cells were used to address the following: improve the ability of MSCs to home to the site of inflammation, and to stabilize the highly variable immunomodulatory properties of MSCs.
The engineered MSCs were studied in cell rolling assay using a P-selectin-coated surface and, in vivo, using a model of inflamed murine ear. Only the MSCs transfected with both PSGL-1 and SLeX exhibited a rolling response under shear stress. In vivo, the double transfected PSGL-1/SLeX MSCs continued to exhibit a rolling response when examined via intravital confocal microscopy.
Homing was next examined in a model in which the mice were sublethally irradiated with the inflamed ear. Although it was unclear why the authors used sublethal radiation, one assumed that the method was intended to eliminate endogenous immune response in order to dissect the effect of the engineered MSCs. The PSGL-1/SLeX MSCs homed more effectively than native MSCs to both the non-irradiated and irradiated mice. In vivo, the PSGL-1/SLeX MSCs exhibited a statistically significant increase in homing as compared to native MSCs at 2 hours post injection. This difference was similar at the later time point, indicating that homing of PSGL-1/SLeX MSCs was relatively rapid. The IL-10 within the triple transfected PSGL-1/SLeX/IL-10 MSCs did not affect rolling or homing to the site of inflammation. However, the combined approach of modulating homing capabilities and immunosuppressive properties of MSCs effectively improved the impact of MSCs on inflammation.
The authors’ conclusion was based on their thoughtful series of investigations using engineered MSCs. However, the conclusion required additional studies with the transfected MSCs. It is unclear why the study examined homing within an irradiated bone marrow since irradiation would not typically cause inflammation to the bone marrow. Additional space within the bone marrow following radiation could account for the increase in homing to the bone marrow but this does not seem relevant to the goals of this study.
The mice used for the in vivo aspects of the study were C57BL/6, yet they were being injected with transfected human MSCs. Although the authors point out that graft rejection was not a main concern because they focused on the short-term impact of the MSC transplant, it cannot be ignored that the act of injecting mice with human MSCs would likely have an impact on levels in inflammation.
The findings have hope for future therapies for inflammation. This would include studies to study the dose of required MSCs and also, to study the model in varied types of inflammation.
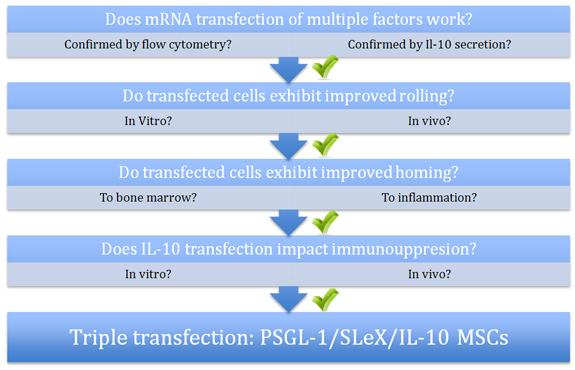
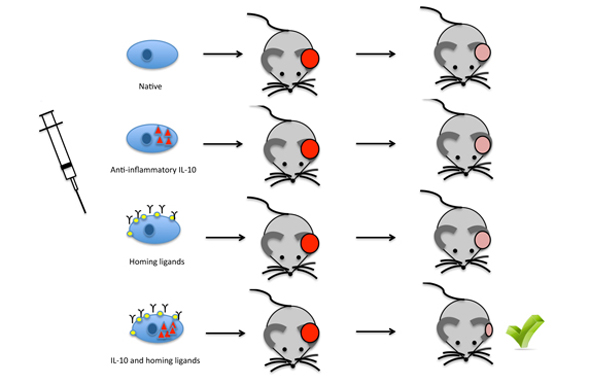
Figure Legend:
After mRNA transfection was confirmed by flow cytometry, PSGL-1/SLeX double transfected MSCs exhibited improved rolling (in vitro and in vivo) and homing (to bone marrow and to sites of inflammation) over native and single transfected MSCs. After being confirmed by functional analysis, IL-10 transfection alone also led to an increase in immunosuppression (in vitro and in vivo). The largest decrease in inflammation in the murine ear was observed with the triple transfected PSGL-1/SLeX/IL-10 MSCs.
|
