(Back)
Jung Bok Lee, Tamra E. Werbowetski-Ogilvie, Jong-Hee Lee, Brendan A. S. McIntyre, Angelique Schnerch, Seok-Ho Hong, In-Hyun Park, George Q. Daley, Irwin D. Bernstein, and Mickie Bhatia. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells. Blood 2013, 122 (7): 1162-73
Summarized by: Everett Henry, Fall 2014)
LAYMAN’S REVIEW
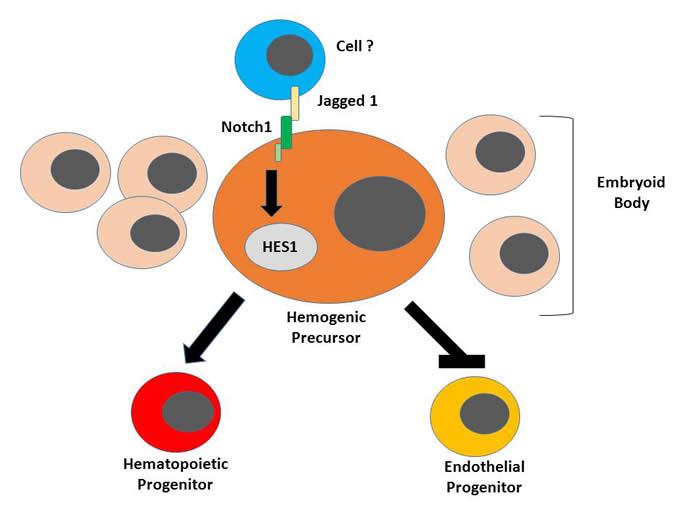
The Notch pathway or signaling is a system cells use to control growth and development from an unspecialized cell state to a more specialized form that performs a unique activity. It consists of several receptors such as Notch1 and Notch2 present on the cell surface that bind specifically to certain proteins namely, Jagged 1 (Jag1) and Delta1 (Dll1). Upon binding, signals are transmitted into the cell and depending on the type of signal the cell changes accordingly. It has been shown that this pathway is involved in the development of blood from precursor cells, referred to as hematopoiesis, and endothelial cells in both vertebrates and invertebrates. In this paper, the investigators demonstrate that Notch signaling drive the formation of blood cells while preventing endothelial cell formation of human precursor cells that can become either type.
They used human embryonic stem cells (hESCs) and stem cells derived from human dermal fibroblasts (hFiB-iPSCs), collectively called human pluripotent stem cells (hPSCs) for their experiments. Growing these cells for 10 days can produce hemogenic precursor cells which are able to differentiate into either blood or endothelial cells. In their first set of experiments, they found that adding Jag1 and Dll1 to the hPSCs, increased the amount of blood cells developing from them and also the amount of hairy and enhancer of split 1 (HES1). HES1 is a protein that is regulated by the Notch pathway. It was also shown that Notch1 is critical for these effects. Importantly, activating Notch signaling in hemogenic precursors resulted in the production of more blood cells and a reduction of the amount of endothelial cells and the opposite effect was observed by suppressing the pathway. Furthermore, the authors showed that these effects were mediated by HES1.
Overall, the ability to control cell fate is an important finding of this group and is clinically relevant particularly for regenerative medicine. The authors suggest that this may be a source of blood cells for patients. Additionally, they propose that keeping the Notch pathway quiescent may be a way for hPSCs to remain in an unspecialized state.
SCIENTIFIC REVIEW
The main objective was to determine if Notch signaling was involved in the induction and specification of hematopoiesis derived from human pluripotent stem cells (hPSCs). Cell fate and proliferation are among the many cellular functions that are regulated by the Notch signaling. Prior seminal work has demonstrated the importance of this conserved pathway for vascular development, hematopoiesis and endothelial cell specification in invertebrates, mice and human hematopoietic cells. In this study, the authors have identified a previously unknown role of Notch signaling in hematopoietic and endothelial cell commitment during early human development.
For their experiments, they used human embryonic stem cells (hESCs) and induced pluripotent stem cells derived from human dermal fibroblasts (hFib-iPSCs) which give rise to bipotent hemogenic precursors. Firstly, they cultured hESCs and hFib-iPSCs with matri-gel immobilized Notch ligands Jagged 1 (Jag1) or Delta1 (Dll1) and found that there was an increase in the number of CD45+ blood cells and hairy and enhancer of split 1 (HES1) expression. Jag1 addition, however had a stronger effect. Additionally, they demonstrated that the Notch1 receptor but not Notch2, using siRNA inhibition, was critical for HES1 upregulation and blood differentiation of the hPSCs.
The involvement of the Notch pathway in early hematopoiesis the authors studied the expressions of HES1 and Notch1 levels in embryoid bodies (EBs) that were stimulated with the ligand, Jag1. Flow cytometry, immunofluorescence and Western blot indicated increases in each protein following the addition of Jag1. Furthermore, the expression of HES1 and Notch intracellular domain (NICD) were detected only in day 10 EBs but not at the later time point, which was studied at day 15. This temporal increase correlated with development of hemogenic precursors and highlighted the significance of Notch signaling during hematopoietic development in the embryo.
The next series of experiments focused mainly on the day 10 embryoid bodies which the authors designated, bipotent hemogenic precursors. The studies elucidated the mechanism of early hematopoietic development by focusing on the role of HES1. This was studied by regulating Notch signaling and HES1 expression. HES1 was either overexpressed or knockdown and Notch, activated with Jag 1 or suppressed with gamma secretase inhibitor (GSI). Notch 1 activation resulted in increased hematopoiesis. HES1 was shown to be involved in Notch signaling during hematopoietic commitment. Interestingly, it was demonstrated that there was a decrease in endothelial specific markers and genes during Notch activation and the effects were reversed upon Notch inhibition. These observations were consistent for hemogenic precursors derived from both hESCs and hFib-iPSCs even at the clonal level.
This work highlights a potentially therapeutic application of hPSCs to generate hematopoietic cells. However, it also reveals that treating pregnant cancer patients with Notch inhibitors such as GSI may pose a health risk to the developing fetus as it may prevent normal blood development. Lee et al. indicate that their work shows that hPSC cell fate is controllable which is of great interest in the regenerative medicine field. Moreover, they suggest that in hPSCs a quiescent Notch pathway is critical to maintaining their pluripotency.
(Back) |
