Reference: Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV
(CCN3) Functions as a Regulator of Human Hematopoietic Stem Cell or Progenitor
Cells. Science 2007; 316:590-593.
Summarized by: Kristine N. McDonald and Jason M. Aptaker
LAY SUMMARY
This summary relates to the role of the Nephroblastoma gene (NOV
CCN3) on the functions of Hematopoietic Stem Cells (HSCs) and their
progenitors. HSCs are present in the adult bone marrow close to the endosteum
where the oxygen levels are low. HSCs can self-renew, which provide them with
the ability to maintain their total numbers. HSCs demonstrate pluripotency which
allows them to differentiate into specialized cells of the immune and blood
systems. Hematopoiesis is the process by which blood cells are generated from
HSCs. In contrast to HSCs, the progenitors are committed cells and have
therefore lost their stemness or pluripotent properties. Due to their limited
ability to form multiple lineages, they are referred as multipotent cells.
The NOV protein is found in human sera and has been linked to
wound-healing and angiogenesis for the growth of blood vessels; it is an
essential regulator of HSC’s and progenitor cells. The presence of NOV is an
indication that cells are in the early stages of differentiation. The
experimental approach showed the highest amount of NOV at the early stage of
differentiation. A series of experiments done in culture showed that the
knockdown of the NOV gene (NOVi), results in a decrease in function,
transplantability, and engraftment. With the impaired gene, cells were smaller
and did not survive for a long period. However, when the amount of NOV gene was
increased (NOV-H), cells had a higher expression of function than the wild type
(NOV) and there was an increase in engraftment and transplantability.
A decrease in the NOV expression affects the functions of HSCs and
progenitors. These defects are demonstrated by the changes in their
intracellular signaling and cycling processes. Such findings demonstrate the
importance of the NOV gene in regulating hematopoiesis.
Further studies on the NOV CCN3 gene may lead to a deeper understanding
of the biology of HSCs and their progenitors. The implications are vast since
HSCs and their progenitors are critical to maintaining a competent immune system
and also to ensure acceptable level of erythropoiesis for red blood cell
formation.
SCIENTIFIC SUMMARY
Hematopoietic stem cells (HSCs) represent the population of self-renewing
multipotent cells that sustains the production of blood throughout life. HSCs
and their progenitors are clinically responsible for the success of bone marrow
transplantations. Nephroblastoma Overexpressed (Nov, CNN3) is
an endogenous gene believed to be a regulator and marker of the differentiated
state. Nov is expressed at the RNA and protein levels in primitive
hematopoietic cells. The Nov gene was stably knockdown in primitive
CD34+ cells with short-hairpin RNA oligonucleotides inserted in a lentiviral
vector with the GFP gene. The knockdown referred to as Novi, exhibits reduced
Nov protein expression as confirmed by RT-PCR in cell lines and cord blood
CD34+.
The functions of the knockdown cells were studied by limiting dilution
analyses with the long-term culture-initiating cells (LTC-ICs), which is an in
vitro assay to study the most primitive hematopoietic progenitors. The results
showed functionally impairment in the knockdown cells (Gupta et al. 590).
Knockdown of Nov also showed diminished expressions of two self-renewal genes, Bmi1 and Gfi1. Nov is reported to be a regulator of Notch,
which is important in hematopoietic regulation. The authors observed that in the
Novi knockdown hematopoietic cells Hes1, a reporter of Notch activity, was
severely impaired.
Clonogenicity was tested using colony-forming cell (CFC) assays. While
only a modest increase in colony number was reported, the cells from this
primary colony were incapable of generating secondary colonies. It seems that
the Novi knockdown’s primary colony after time lost its ability to self-renew
and repopulate. Multiple mechanisms for failure of the Novi cells to engraft
were postulated. There was a reduction in primitive CD34+ CD38- lin- cells,
increased time in the G2/M [Mitosis] phase, and a reduction in p21 expression.
The absence of Nov may cause of diversion of fate towards differentiation.
The in vivo capabilities of Novi were tested using xenotransplantation
for hematopoietic reconstitution in NOD/SCID mice. CD34+ CD38- cells infected
with Novi or control were transplanted in NOD/SCID mice as assessed for
engraphment at 8 weeks. Quantification of viral sequences in the DNA of human
cells in recipient bone marrow was found to be a factor of 22 lower then in Novi
than that of control. In order to corroborate, a second transplantation
experiment was performed using a cytomegalovirus (CMV) to form a spleen
focus-forming virus to create slx-Novi viruses and an empty vector control.
Assessment of engraphment was found 14 times lower in the slx-Novi recipients
then the empty vector controls. It is concluded that Nov is required for
stem-cell dependent engraphment in vivo.
Gain of function studies were conducted to evaluate if increasing Nov
levels would enhance stem/progenitor activity. A recombinant human Nov protein
(rhNov) was purified and added to CFC assays causing a doubling in colony
formation and enhanced replating. This change occurred in the multipotent cells
while it had no effect on the committed progenitors. Next cord blood cells were
expanded in various factors [Flt-3, stem cell factor, thrombopoietin, IL-6] with
or without rhNov. Increased colony formation was found for all rhNov exposed
versus non-rhNov cells. Exposure to rhNov also reverses the functional deficit
that Novi cells exhibited in secondary replating experiments. In vivo Nov-hi was
compared to control through xenotransplantation into SCID mice measured by GFP
fluorescence. Concluded was that high levels of Nov expression conferred a
selective engraftment advantage. A second transplant was performed removing the
Nov-hi cells from the primary entrapped animal cultured in vitro for 4 weeks
then transplanted to a secondary recipient. Five out five subjects had
confirmed engraphment using RT-PCR for DNA from bone marrow.
Figure 3 summarizes the discussed study, which identifies Nov in humans
as an essential regulator of HSCs and their progenitors. Levels of Nov can
impact the self-renewal and repopulating ability of the HSCs and progenitors.
Some questions arise in the ability of this study to precisely identify these
cells as stem cells. The incorporation of virus into the CD34+ cord blood cells
may contribute to the long-term culture ability in vitro of LTC-ICs. The
measurement of the Nov-hi expression is measured by fluorescence and not by FACS
and cell markers. Further experimentation needs to investigate Nov expression on
truly identified functional HSCs.

Figure 1. Nov is needed for HSC and Progenitor
Cells to differentiate into different types of blood cells.
Less
differentiated More
differentiated

Figure 2. The amount of NOV is the highest in cells that are
less differentiated such as HSC and Progenitor Cells.
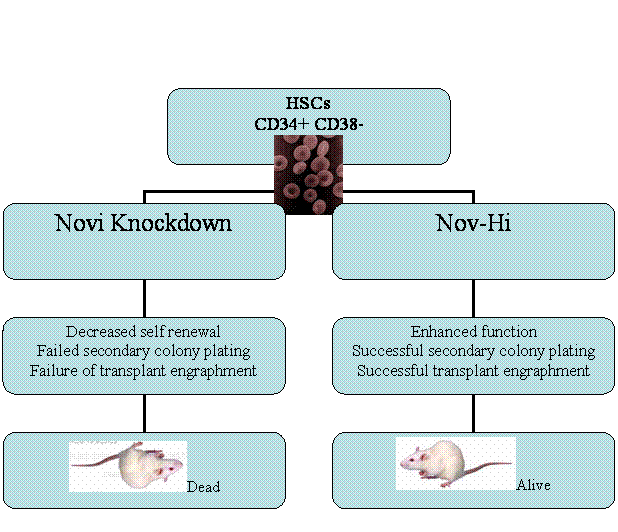
Figure 3.
