Abad M, Mosteiro L, Pantoja C, Cañamero M, Rayon T, Ors I, Graña O, Meǵıas D, Doḿınguez O, Mart́ınez D, Manzanares M, Ortega S, Serrano M. Reprogramming in vivo Produces Teratomas and iPS Cells with Totipotency Features. Nature 2013, 502: 340-344.
Summarized by: Owen Sweeney, Fall 2014
Layman’s Review
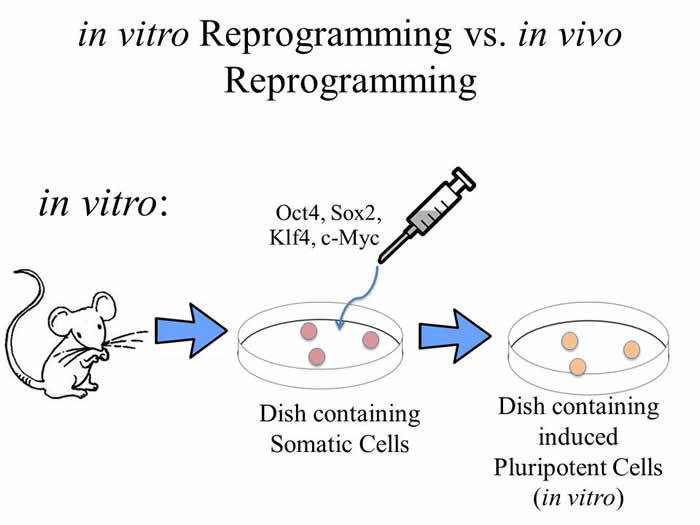
Mature cells are very different from each other, and can be classified by their ‘lineage’. This lineage, or line of cells from which one cell comes from, share many characteristics with each other, and generally travel from progenitor to fully differentiated cell in the body (in vivo). However, under certain conditions, a fully differentiated cell can revert back to become a progenitor cell. If done completely, a cell type can revert all the way back to its original cell type, a stem cell. These cells give rise to the different lineages of an organism, such as blood (hematopoietic) cells, organ lining (epithelial) cells, stomach cells, brain cells, skin cells, etc. This is important because if a cell population in your heart is damaged and unable to repair itself, it is possible to take another cell type, revert it back to its ‘stem cell’ state, and then differentiate into heart cells to help with recovery. While this process is well studied as a lab technique, in vitro, several studies have indicated that this phenomenon might be able to occur in a living organism, in vivo, under the right circumstances and induced chemically.
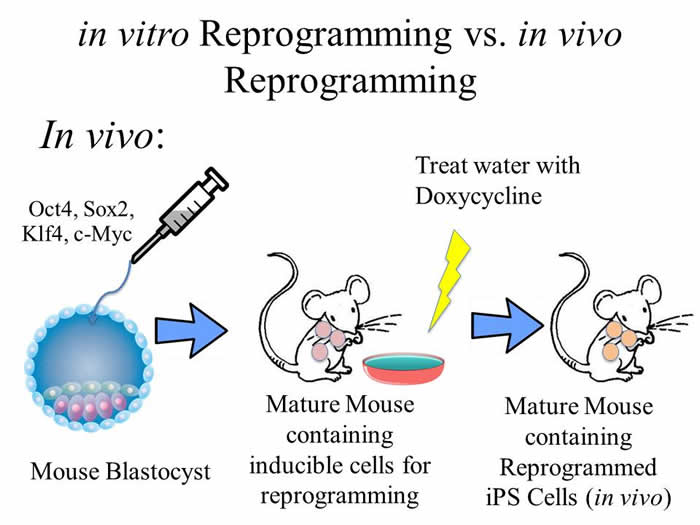
The scientists took blastocysts (sack of cells that becomes a full organism for birth) of mice and injected them with the proper reprogramming factors. After development and maturity of the mice, the mice were fed a chemical meant to induce the reprogramming of the cells. The scientists tested for success by observing the organs of the mice and searching for specific tumors, called teratomas. These teratomas are tumoral masses formed from these induced stem cells. The teratomas were present, which proves that in vivo reprogramming of cells is possible.
The scientists also showed that the cells that resulted from in vivo reprogramming were much like stem cells from early organismal development, embryonic stem cells (ES cells). Although the science of the paper is excellent, the value of this type of work is unclear.
Scientific Review
Reprogramming cells in vitro into a pluripotent state is a well-researched phenomenon that opens doors to many therapeutic opportunities, including tissue regeneration and improved bone marrow transplantation. However, previous studies have shown that, in vivo, the seemingly irreversible direction of differentiation can be altered, and one cell type can be reprogrammed into another. The authors hoped to advance the technique of in vivo reprogramming of cells and to study the cell types conferred upon reprogramming. The authors used animal models to study in vivo reprogramming with in vitro comparison studies with iPS cells (induced pluripotent stem cells) and ES cells (embryonic stem cells). Studies tested plasticity, pluripotency by phenotype and differentiation lineages.
Four transgenic mouse lines were obtained that contained a transcriptional activator and the four genes required for reprogramming (Oct4, Sox2, Klf4, c-Myc, referred as OSKM). The OSKM genes were taken up by the mouse embryonic fibroblasts (MEFs) using a lentiviral cassette. The genes were controlled by induction with doxycycline. Two of the four mouse lines were successfully reprogrammed, resulting in two lineages (i4F-A and i4F-B) of mice that were selected as candidates for further investigations.
The mice were treated with doxycycline for 1 week to induce in vivo reprogramming. These in vivo iPS cells expressed the pluripotent markers and, when removed and cultured, showed evidence of pluripotency. In line with reprogramming, there were tumoral masses formed in the kidneys, pancreas, intestine and stomach. Several of the masses were shown to be teratomas with cells of the three germ layers, indicating in vivo reprogramming of cells. After reciprocal transplantation of bone marrow (BM) between wild type (WT) mice and i4F mice, both sets of BM-reconstituted mice developed multiple teratomas. This suggested that both hematopoietic and non-hematopoietic cells are reprogrammable in vivo. Double immunohistochemical studies identified CK19 (epithelial marker) and NANOG (pluripotency marker) in the teratomas, suggesting that reprogramming occurred in situ within the epithelium. The results also showed that reprogrammed hematopoietic cells were found in the circulation.
Analysis of the transcriptomes of the three cell types (in vivo iPS cells, in vitro iPS cells, ES cells) revealed a much closer similarity between the in vivo iPS cells and the ES cells, compared to any other pairing. Also, in vivo iPS cells showed lineage markers of the trophoectoderm, and with the in vivo iPS cells forming embryo-like structures. This finding was novel because the iPS and ESC did not similar findings.
This paper was very clear in its objective, to show reprogramming in vivo is possible, and direction of the experiments, to compare the in vivo iPS cells to other stem cell types.
The significance of the work was unclear. The formation of teratoma makes it difficult to understand how the technique could be used in vivo. Regarding application, it is unclear if the in vivo reprogramming has an advantage over in vitro reprogramming. Undoubtedly, the risks of teratoma formation during the reprogramming would be mostly harmful to human patients, even if the reprogramming can be localized to a few cell types or tissues.
|
