Benjamin D Cosgrove, Penney M Gilbert, Ermelinda Porpiglia, Foteini Mourkioti, Steven P Lee, Stephane Y Corbel, Michael E Llewellyn, Scott L Delp & Helen M Blau. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nature Medicine 2014, 20 (3): 255-264.
Prepared by: Piotr Pobiarzyn, Fall 2014
Layman’s Review
Our muscles have the natural capability to regenerate when damaged. The main cells involved in the regeneration are the Satellite Cells within the skeletal muscles (one’s that help you move your arms, legs etc.). As one age, the body’s ability to regenerate and repair damaged muscles diminishes. This has been attributed to the satellite cells (also known as Skeletal Muscle Stem Cells) losing their “vigor”, simply put they are not as effective as their young counterparts.
Scientists have investigated this problem on mice, estimating that in aged mice only 1/3 of Satellite cells are still able to fulfill their task, to repair and regenerate. So what had happened to the remaining 2/3? Researchers observed that they contain elevated levels of certain cell regulators, p38 alpha and p38 beta, responsible for cell’s activity. Next, the logical step was to ask - if those upregulated levels can be brought lower to enhance the Satellite cells regenerating abilities. In order to do so, a specific inhibitor for p38 alpha and p38 beta was used to block their action.
The satellite cells were collected from the aged mice, treated with the inhibitor and reintroduced into the aged mice. The effect was promising but still not enough to call it significant. In order to enhance the activity of the aged satellite cells, researchers decided to mimic the natural environment of the satellite cells, namely to create a surface that would imitate a muscle, where the satellite cells reside. They used a hydrogel, a specialized gel –like-material used as a scaffold, which was adjusted to resemble the surface of a muscle, its hardness and texture. Next, the collected satellite cells were treated with the inhibitor with the hydrogel used as a matrix. Once such cells were reintroduced into the aged mice the effects were staggering. The Satellite cells ability to repair and regenerate damaged muscles were as good as in young mice.
These results carry a great potential in perspective of human applications – where elderly patients can be helped to boost their healing process after muscle related injuries, or to simply enhance their muscle function.
Scientific Review
The main objectives of the study was to determine the underlying causes of the decline in Skeletal Muscle Stem Cells (MuSCs) regenerative and self-renewing properties in aged muscles and propose a method to restore these essential functions.
Transplantation of MuSCs derived from aged and young mice showed a 2/3 decrease of functional stem cells in aged mice MuSCs. Since there was minimal effect by the young microenvironment the outcome of the transplant studies indicated autonomous cell-intrinsic defect of the aged MuSCs. In order to understand this intrinsic defect, the authors used FACS-purified MuSCs from aged and young mice and analyzed the expression of senescence associated markers, p16Ink4a and p21Cip1 (cell-cycle inhibitors). They observed a significant increase of these markers in the aged cells as compared to those from young mice. Further studies indicated that the senescence markers could be induced by persistent activation of cellular stress-related signaling pathways with significantly higher expression of p38α/β Mitogen activated protein kinase (MAPK) in MuSCs from aged mice as compared to cells from young mice. The findings were applied to determine if the findings could be translation by targeting p38α/β MAPK signaling.
Further studies to rejuvenate the MuSCs from aged mice were conducted by combination of biophysical and biochemical approaches. Regarding biochemical approach, the authors tested an imidazole-based ATP competitive inhibitor of p38α/β MAPK signaling, SB202193 (SB) whereas for biophysical component used a soft hydrogel matrix which mimics the rigidity (12kPa) and texture of skeletal muscle (equivalent of laminin protein). MuSCs from aged mice when cultured on hydrogel and treated with SB, displayed significantly reduced expression levels of p38 α/β signaling (inhibition or siRNA), even lower than the expression observed from MuSCs of young mice with similar treatment. There was a 35-fold increase in the proliferation of age-derived cells as compared to similar treatment from young mice. The expressions of Pax7 (muscle stem cell gene) and Myog (encoding myogenin – a commitment/differentiation inducer) genes in the cells treated with SB on hydrogel indicated an increase in Pax7 and decrease in Myog, indicating that proliferation was enhanced but this occurred sustained multipotency.
The authors finally studied the regenerative implications for their findings. MuSCs from aged and young mice treated with SB on hydrogel were transplanted into the hindlimbs of 2-months old immune deficient mice. Transplant engraftment frequency was measured by Bioluminescence imaging (BLI). Cells cultured on hydrogel alone (-SB) displayed a significant difference between MuSCs from aged and young mice (5% and 34% respectively). Transplantation and treatment with p38 α/β inhibitor showed a substantial increase in engrafted MuSCs from old mice with a relatively smaller rise noted for MuSCs from young mice.
The study was conducted meticulously and included numerous alternatives and controls to validate the findings. The combination of biophysical and biochemical approaches, the authors displayed a great insight towards the complexity of muscle regeneration. The findings bring a promising treatment for human diseases, in particular the aging individuals.
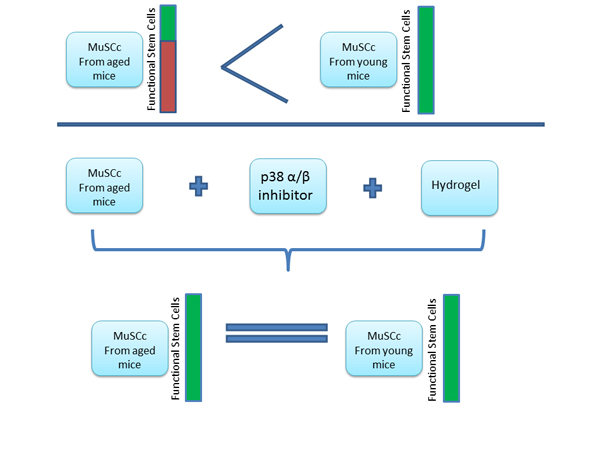
Figure 1. (Diagram)
Transplant analyses of Muscle Stem Cells (MuSCs) from aged and young mice show a 2/3 decrease of functional stem cells in aged mice stem cells. The MuSCs from aged mice were collected and plated ex-vivo on hydrogel (to mimic the skeletal muscle surface structure) and treated with SB (p38α/β MAPK Inhibitor), which restored function of MuSCs
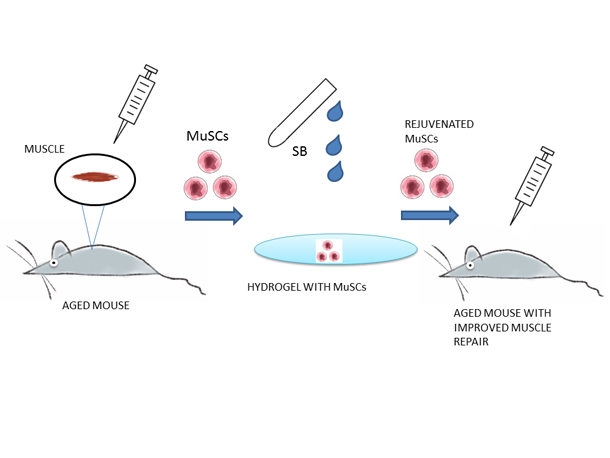
Figure 2 (Cartoon)
Muscle Stem Cells from aged mice were collected and plated ex-vivo on hydrogel (to mimic the skeletal muscle surface structure) and treated with SB (p38α/β MAPK Inhibitor). After the treatment MuSCs were reintroduced into the aged mice enabling the regeneration of muscles comparable with the young mice.
(Back) |
