Back
Reference: Yamada Y, Yokoyama S-I, Wang Z-D, Fukuda N,
Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce
bone marrow nonhematopoietic cells to differentiate into cardiomyocytes.
Summarized by: Tara Gooen and Edward Vallejo, Fall 2007
LAY REVIEW
Introduction
Researchers have been working on a way to repair damaged cardiac
tissue after a heart attack. Altering genes and transplanting stem cells into
the affected area are two methods that show the most promise of working. This
paper focuses on a group of newborn fat cells which could be a source of cardiac
cells that could be used for the repair process. The authors also see whether
mesenchymal (bone) stem cells when compared with the hematopoietic (blood and
immune) stem cells are better at becoming these cardiac cells.
The cardiac cells were formed by growing two different types
of cells together. To show that they were not formed because of fusion between
the two different cell types, isolated and cultured bone marrow cells, including
both mesenchymal and hematopoietic stem cells, were injected into the hearts of
mice that had just suffered a chemically induced heart attack.
Surface Characteristics of Possible Cardiac Stem Cells in
Fat Tissue
Fat tissue was removed from unborn and newborn mice and the fat
cells were analyzed for three cell surface markers (CD45, Ter119, and CD31) by
flow cytometry, a cell sorting technique. Cells that did not express those
markers were further examined for expression of three other markers, (c-kit,
Sca-1, and CD133). Cardiac cells were then formed from each of these cell
populations and it was observed that those cells that positively expressed the
CD133 marker were the best at becoming the cardiac cells because they possessed
cardiac markers on their surface. Studies involving heart drugs also showed that
these cells not only had the surface properties, but also the functional
capability of cardiac cells since they responded just like cardiac cells would
to those drugs.
Newborn Fat Cells Cause Cardiac Cell Formation from Bone
Marrow Cells
Previous studies have shown that in order for the bone marrow cells to
spontaneously develop into cardiac cells, the right microenvironmental factors
were needed. The authors’ experimental model achieved this by culturing bone
marrow cells from one type of mice, whose cells expressed a green fluorescent
protein when viewed under the microscope, with CD133+ newborn fat cells from
mice that did not have this protein for 14 days. These two types of cells were
directly touching one another and developed into cardiac
cells.
Bone Marrow Stem Cells Cultured with Newborn Fat Cells
Developed into Cardiac Cells without Fusing with Each Other
To show that these cardiac cells did not develop because of the bone marrow
stem cells fusing with the newborn fat cells, the two were separated from each
other by a 0.4 μm membrane. This physical barrier had small openings in it to
allow soluble factors to pass between the two cells to mimic the
microenvironment around the cells that would normally be present. The authors
concluded that the newborn fat cells did not fuse with the newly formed cardiac
cells. This was because cell signals that were only seen in each cell type
before the beginning of the experiment were still observed at the same strength
after 14 days. They also learned that the potential to form cardiac cells was
not as strong in the indirect contact model as in the direct contact model.
Cell Contact Is Critical for the Converting Bone Marrow
Stem Cells into Cardiac Cells
As the authors established in the previous section, bone marrow
stem cells did not just fuse with the newborn fat cells to appear like cardiac
cells. However, the efficiency of bone marrow stem cells converting into cardiac
cells was only 20% when the bone marrow and newborn fat cells were not in direct
contact. The authors then chemically fixed the newborn fat cells to prevent any
communication via soluble factors. This did not stop the conversion. Therefore,
the authors concluded that there might be two independent mechanisms in the
conversion: (1) factors which the cells secrete that do not need direct contact
between cells and (2) cell-to-cell surface communication which does require
direct contact. Further, the authors showed that an important calcium dependent
cell-to-cell surface communication mechanism is instrumental in the conversion.
Newly Formed Cardiac Cells Contributed to Heart
Regeneration
The authors then studied the usefulness of these cardiac cells in
mice. First, bone marrow stem cells were cultured with the newborn fat cells.
The bone marrow stem cells showed conversion factors after only 5 days. The
bone marrow cells were then isolated and cultured for another 14 days. Those
cells were injected into the hearts of mice which were suffering from a
chemically induced attack. The authors observed several improvements in the
hearts of mice that received the cardiac cells, including the communication of
these cardiac cells with the existing cardiac cells.
Mesenchymal Stem Cells in the Bone Marrow Are a Major
Source of Newly Formed Cardiac Cells
Since bone marrow stem
cells are a mixed population of cells, including mesenchymal (bone) stem cells
and hematopoietic (blood and immune) stem cells, the authors studied which type
of stem cell converted to cardiac cells better. It was found that there was a
20-fold difference in the conversion of mesenchymal stem cells when compared
with the conversion of hematopoietic stem cell.
Summary
The paper further demonstrates
that the authors may have discovered a source of cardiac stem cells. However,
the use of a mixed population of bone marrow stem cells raises unanswered
questions. We would like to see similar experiments performed with only
mesenchymal stem cells, to study the efficiency of conversion and what specific
factors are necessary. In this paper, the authors have further shown the
possibility of CD133+ newborn fat cells as being a source of cardiac stem cells.
There are many possibilities for further research.
SCIENTIFIC REVIEW
Introduction
The repair of damaged cardiac tissue following myocardial infarction to the
level of viable functionality has been the goal of researchers in recent years.
Two important areas of investigation that show the most promise for generating
positive results include genetic manipulation and stem cell transplantation. The
primary focus of this paper is experimentation with a specific population of
cells in Brown Adipose Tissue (BAT) that could most likely be a source of
Cardiac Stem Cells (CSCs). Additional experiments also give evidence to support
whether MSCs or HSCs from a bone marrow mixed population are better at
differentiating into cardiomyocytes (CMs). Effective demonstration of an in vivo
infarction model attempts to show that the mechanism of CM induction and repair
through coculturing is not through cell fusion, but through bivalent
cationmediated cell-to-cell contact between cells.
Surface Phenotype of CM Progenitors in BAT
Brown Adipose Tissue was dissected from neonatal and postnatal mice and
analysis was carried out on the Brown Adipose Tissue Derived Cells (BATDCs) for
three cell surface markers by flow cytometry (CD45, Ter119, and CD31). Among
cells negative for the aforementioned markers, positive expression of the
markers c-kit (1.4%), Sca-1 (16.6%), and CD133 (3.5%) was observed. From each of
these respective populations, CMs were derived and analyzed using
immunocytochemical analysis and transmission electromicrographs. Of these,
CD133+ cells were shown to be the best at differentiating into CMs based on
criteria such as surface adherence and positive expression of known CM markers
such as SA, GATA, Troponin T and Troponin I.
In addition, pharmacological studies of this specific cell population show
that they exhibit CM-like responses to medication in both an agonistic and
antagonistic manner. Overall, these results provide evidence that not only do
these differentiated cells express phenotypic markers of CMs, but they also
possess their functional capabilities as well.
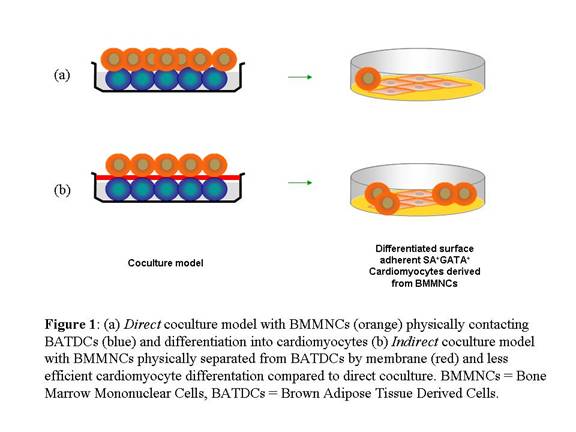
BATDCs Effectively Induce CM Production from BMCs
Previous in vitro studies showed that although Bone Marrow Cells (BMCs) were
a source of CMs, they could not spontaneously differentiate into CMs. In order
to produce these results, the appropriate microenvironmental cues were required.
The experimental model accomplished this through 14 days of coculturing of Bone
Marrow Mononuclear Cells (BMMNCs) from green fluorescent protein mice with
CD133+ BATDCs from ROSA 26 wild-type mice. These two types of cells were in
direct surface contact with one another and produced differentiated, surface
adherent CMs.
BMMNCs with BATDCs Differentiated into CMs Without Fusion
Mechanism
In order to show that CM development was not due to fusion between the BMMNCs
and BATDCs, the two cell types were physically separated by a 0.4 μm membrane.
This allowed the passage of microenvironmental cues such as cytokines between
the cells while simultaneously maintaining a strict physical boundary. Signals
distinct to both cell types were shown by RT-PCR to still be differentially
expressed 14 days after coculturing. This led the authors to conclude that
fusion did not play a role in CM differentiation. However, differentiation
potential in the indirect coculturing model was not as efficient as it was in
the direct contact experiments.
Cell Contact Mediated by Bivalent Cation Is Critical for the
Differentiation of BMMNCs into CMs
As the authors established in the previous section, it was seen that cell
fusion is not the only factor in the differentiation of BMMNCs into CMs. However
the efficiency of differentiation from BMMNCs to CMs without direct contact
coculture was only 20% of the differentiation when compared with with direct
contact coculture between BATDCs and BMMNCs. To further investigate the
possibility of cell fusion as a major factor in the differentiation, the authors
demonstrated that BMMNCs differentiated into CMs after chemically fixing the
cell surface of the BATDCs with paraformaldehyde.
Additionally, the investigators studied cadherin-mediated
calcium dependent cell-to-cell contact in the regulation of BMMNC
differentiation in CMs. Through the use of calcium chelator EDTA or EGTA, the
authors showed that all differentiation of BMMNCs to CMs is suppressed.
Further, via anti-Ecad antibody or soluble cadherin, the authors showed that
Ecad-mediated cell-to-cell contact is also a key component in the
differentiation of BMMNCs. This suggests that there might be two independent
ways of inducing CMs from BMMNCs: secreted factors and membrane
proteins.
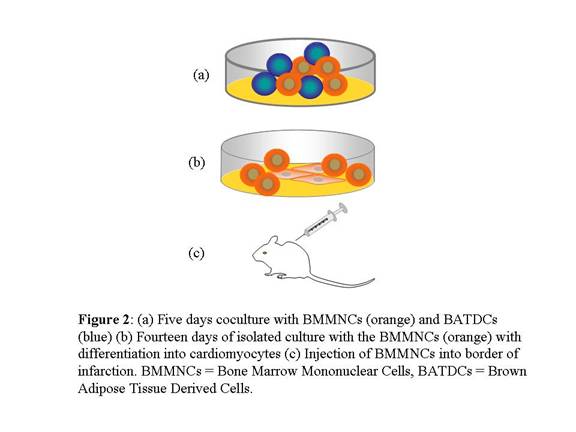
Educated BMMNCs Contributed to CM Regeneration
The
authors then performed in vivo studies in murine models. First, it was
determined that BMMNCs showed a commitment into CMs after only 5 days of
coculture culture with BATDCs when followed by 14 days of isolated culture.
About 9.7% of BMMNCs showed CM lineage markers. The authors then injected these
differentiated BMMNCs into the border of infected hearts of mice after the
induction of an acute myocardial infarction. They observed significant
improvement in the mice that received the transplanted cells, such as the
formation of gap junctions, intercalated disks, and secretions of cardiac
myocyte-specific factor. Further, the authors showed that the differentiated
BMMNCs had not undergone cell fusion after implantation via FISH staining.
Nonhematopoietic Cells in the BM Are a Major Source of
CM
To determine what type of cells are differentiating into CMs, the authors
sorted the differentiated BMMNCs cells by lineage markers and determined that
there was a 20-fold difference in the differentiation of nonhematopoietic stem
cells (CD34-, CD31-, CD105+, mesenchymal) versus hematopoietic stem cells (Lin-,
c-kit+).
Discussion
The paper further demonstrates, in conjunction with the previously published
paper, that the authors may have discovered a source of cardiac stem cells.
While the cell fusion experiments are useful, the use of a mixed population of
BMMNCs raises unanswered questions. It would be nice to see the same
experiments performed with only mesenchymal stem cells to determine the
efficiency of differentiation and determine if there are additional factors in
the mixed population that are not directly associated with BMMNCs.
Additionally, the determination of which factors the BATDCs actually provide to
induce BMMNC differentiation would be very interesting. In this paper, the
authors have further shown the possibility of CD133+ BATDCs as being a source of
cardiac stem cells. There are many possibilities for further research.
ACKNOWLEDGEMENTS
This review was prepared by
the following graduate students, Advanced Stem Cell Seminar (Fall 2007),
University of Medicine and Dentistry of New Jersey, Graduate School of
Biomedical Sciences:
Tara Gooen and Edward Vallejo
Course Instructor : Pranela Rameshwar,
Ph.D.
Back |
