Primary NK and CAR-NK expansion from PBMC or CBMC
(Minh Ma, Yan Yang, and Dongfang Liu. 09/15/2020)
Materials
- PBMC isolated from healthy donors - frozen in freezing medium (90% FBS + 10% DMSO)
- Feeder cells – irradiated 221mIL21 (made in-house) - frozen in freezing medium (90% FBS + 10% DMSO)
- 6-well G-REX plate (Wilson Wolf, Cat #80240M)
- R-10 media – RPMI (VWR, Cat #45000-404) containing 10% FBS (Corning, Cat #35-010-CV), 1% Penicillin: Streptavidin solution (VWR, Cat #45000-652), and 1% L-glutamine (VWR, Cat #45000-304)
- D-10 media – DMEM (VWR, Cat #45000-304) containing 10% FBS (Corning, Cat #35-010-CV) and 1% Penicillin: Streptavidin solution (VWR, Cat #45000-652)
- Non-treated 24-well plate (Fisher Scientific, Cat #13-690-071)
- OPTI-MEM (ThermoFisher, Cat #31895)
- Genejuice transfection reagent (VWR, Cat #80511-356)
- IL-2 (Peprotech, Cat #200-02) – final concentration of 200 U/ml
- IL-15 (Peprotech, Cat #200-15) – final concentration of 5 ng/ml
Step-by-Step Procedures, please also read our publication1.
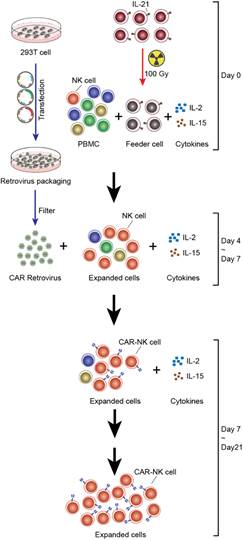
Day 0 – Human Primary NK Cell Expansion
- Thaw frozen PBMCs and frozen irradiated feeder cells in 37oC water bath
- Wash PBMCs and 221mIL21 cells by centrifugation at 400 rcf for 5 minutes with 5-10 ml R-10 media
- Save some PBMCs for flow cytometry (Note: the initial NK cell purity is an important factor in calculating NK cell expansion rate)
- Resuspend cells in 1 ml R-10 media. Count cells using Trypan Blue.
- Mix 5 x 106 cells of PBMCs with 10 x 106 cells of irradiated 221mIL21 cells in a G-REX well
- Add 30 ml of R-10 media supplemented with IL-2 (200 U/ml) and IL-15 (5 ng/ml)
- Incubate G-REX plate at 37oC with 5% CO2
- Change media to maintain NK cells every 3-4 days.
Note: keep less than 20 x 106 cells per well for further expansion at each change. For the best viability, the total cell number in each well should never exceed 100 x 106.
- Record total cell number and viability and perform flow cytometry every 3-4 days to calculate the NK cell expansion rate
Day 1 – 293T cell preparation
- Split 293T cells with D-10 media to 1.8 x 106 cells per 10 cm2 plate
- Incubate 293T at 37oC with 5% CO2
Day 2 – Retrovirus transfection
- In a 1.7 ml Eppendorf tube, mix 470 ml of OPTI-MEM with 30 ml of Genejuice
- In a separate Eppendorf tube, add 2.5 ug pRDF, 3.75 ug Pegpam3, and 2.5 ug CAR construct in SFG vector in OPTI-MEM so that the final volume is 500 ml
- Mix the solutions in steps 1 and 2 together, dropwise
- Incubate at room temperature for 15 minutes
- Add 1 ml of the mixture from step 4 to each 293T plate on Day 1, dropwise
- Incubate plates at 37oC with 5% CO2 for 48-72 hours
Day 3 – Retronectin plate-coating
- Dilute Retronectin protein with PBS to the final concentration of 50 – 60 ng/ml
- Add 500 ml of diluted Retronectin into each well of a non-treated 24-well plate (5 wells per CAR construct)
- Parafilm and incubate plate at 4oC overnight
Day 4 – Transduction
- Centrifuge Retronectin plate at 3000 x rpm for 30 minutes at 4oC. Discard supernatant
- Block each well of the 24-well plate with R-10
- Incubate the plate at 37oC with 5% CO2 for 1 hour
- Pre-warm the centrifuge to 32oC while the Retronectin plate is being blocked
- Collect retrovirus supernatant by filtering the transfected 293T cells with a 0.45 mm filter
- Aliquot 2 ml of filtered retrovirus supernatant into each well
- Centrifuge the 24-well plate at 3000 x rpm for 2 hours at 32oC
- During plate centrifugation, collect the expanded PBNK cells from Day 0 and count the cells using Trypan Blue
Note: continue expanding PBNK cells by adding R-10 media supplemented with IL-2 (200 U/ml) and IL-15 (5 ng/ml)
- Dilute expanded PBNK cells with R-10 media supplemented with IL-2 (200 U/ml) and IL-15 (5 ng/ml) to 0.25 x 106 to 0.5 x 106 cells/ml (0.5 x 106 to 1 x 106 cells per well)
Note: record total cell number, viability, and save some expanded PBNK cells for flow cytometry as these values are important in determining NK cell expansion rate
- After centrifugation, partially aspirate the retrovirus supernatant from each well
Note: DO NOT COMPLETELY ASPIRATE as this will decrease the transduction efficiency
- Aliquot 2 ml of diluted expanded PBNK cells from step 8 to each well
- Centrifuge plate at 600 rcf for 10 minutes at 32oC
- Incubate plate at 37oC with 5% CO2 for 48 – 72 hours
Note: DO NOT PARAFILM the plate
Day 6 or 7 – CAR-NK collection
- Gently collect cells from the 24 well plate and transfer cells to a 50 ml Falcon tube
Note: try not to generate bubbles as this will result in a decrease in cell viability
- Centrifuge tube at 400 x rcf for 5 minutes
- Resuspend pellet with 1 ml R-10 media and count cells using Trypan Blue
Note: save some cells for flow cytometry for the determination of the transduction efficiency
- Transfer the resuspended cells to a G-REX well containing R-10 media supplemented with IL-2 (200 U/ml) and IL-15 (5 ng/ml)
- Incubate G-REX plate at 37oC with 5% CO2
- Change media to maintain NK cells every 3-4 days.
Note: keep less than 20 x 106 cells per well for further expansion at each change. For the best viability, the total cell number in each well should never exceed 100 x 106.
- Record total cell number and viability and perform flow cytometry every 3-4 days to calculate the NK cell expansion rate
- Cells can be used for the appropriate in vitro or in vivo assays
Note: the ex vivo expanded PBNK and CAR-NK cells can be cultured in 37oC incubator for approximately 4 weeks
- NK cell number and purity can be examined at day 7, day 11, day 14, day 18, and day 21 by flow cytometry.
Notes: Use this method for CBMC expanded NK and CAR-NK cells
References:
1. Yang, Y., et al. Superior Expansion and Cytotoxicity of Human Primary NK and CAR-NK Cells from Various Sources via Enriched Metabolic Pathways. Mol Ther Methods Clin Dev 18, 428-445 (2020).

