Research
About the lab
Human cytotoxic lymphocytes include natural killer (NK) cells and cytotoxic T lymphocytes (CTL). Particularly, we are interested in studying the biology of human NK cells.
Specifically, we are applying biochemical, live cell imaging, biophysical approaches, and animal models to study the signaling and functions of the immunological synapses (IS) and human NK cell dysfunction in chronic infectious diseases, such as HIV and HIV-related malignancies, and cancers.
We are using the glass-supported planar lipid bilayer system and single-molecule imaging to study the biology of NK cells, with a focus upon infectious diseases and cancers. Planar lipid bilayers supported on glass coverslips have been used for imaging IS (Fig. 1). We have inserted ligands for CTL or NK cell activation receptor (AR) or inhibitory receptor (IR) into lipid bilayers.
To study the dynamics of IS formation, we label the ligands with fluorescent dyes and imaged under total internal reflection fluorescence (TRIF) microscopy.
To study the degranulation of lytic granules at IS, we monitored the fluorescence signal of lysosome-associated membrane protein 1 (LAMP-1, also known as CD107a) by a directly conjugated Fab of a CD107a mAb in the imaging chamber under TIRF microscopy.
Alternatively, we also used the pH-sensitive green fluorescence protein (pHluorin) fused to the lumenal domain of Fas ligand (FasL) to visualize degranulation events with a high time resolution (Liu D., et al., Immunology & Cell Biology, 2012).
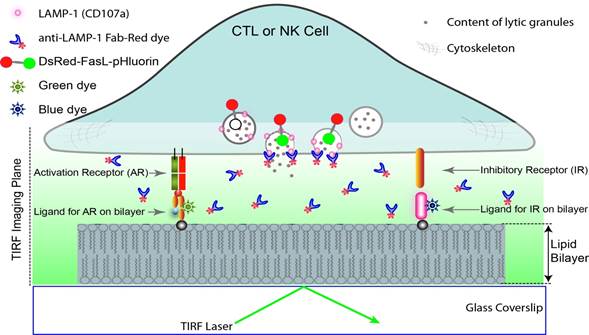
Fig. 1: Combining lipid bilayer system with TIRF microscopy to study the biology of cytotoxic lymphocytes and immunological synapses (IS).
To study the signaling events at IS, we stain NK cells on lipid bilayers with antibodies against signaling molecules or fluorescently tagged molecules by multidisciplinary approaches including single-molecule fluorescence resonance energy transfer (smFRET) and super-resolution stimulated-emission depletion (STED) imaging.
We also use STED study the structure of natural killer (NK) cell immunological synapses on the glass-supported planar lipid bilayer (Peilin Zheng, et al., 2015, JOVE).
https://www.jove.com/v/52502/super-resolution-imaging-natural-killer-cell-immunological-synapse-on

Projects
Project #1: The role of Crk in immune response
Cytolytic lymphocytes, including natural killer (NK) cells and cytotoxic T lymphocytes (CTL), play an important role in the human immune response to infection and malignancy.
How these cells mount an effective immune response to infection and malignancy represents one of the key unsolved problems in immunology today. The main work in our group seeks to identify the mechanism(s) by which a small adaptor protein, CT10 regulator of kinase (Crk), and its receptor-driven phosphorylation control NK cells activation and inhibition (Fig. 2).

Fig. 2: Crk and pCrk control NK cell activation and inhibition. Phosphorylation of Crk induced by inhibitory receptors blocks NK cell activation. Interconversion between un-phosphorylated Crk (green) and phosphorylated Crk (red) functions as a molecular switch in the control of NK cell activation via interaction between Crk and pVav-1 or interaction between pCrk and SHP-1. Modified from Liu, D. Immunol and Cell Biol. (2014).
An essential control of NK cytotoxicity is provided by inhibitory receptors expressing on NK cells. A recent study demonstrated that HLA-E (a ligand for inhibitory receptor, CD94-NKG2A) alone induces Crk (chicken tumor virus no.10 regulator of kinase) phosphorylation in NK cells (Liu et al., Immunity, 2012 and Huang et al., J Allergy and Clinical Immunology, 2018 ). The exact functions of Crk in regulation of immune response have not been explored. We are interested in the role of Crk in immune response by leveraging a common immunodeficiency, such as partial DiGeorge syndrome (Zheng et al., J Allergy and Clinical Immunology, 2015), as well as a novel NK-specific murine knockout system (Nabekura et al., Journal of Immunology, 2018).
The proposed work will bring significant conceptual and technical innovation to a critical and understudied area of NK cell research: the molecular basis of Crk in NK cell activation and inhibition.
Project #2: Immunotherapy for HIV and HIV-related malignancies
As patients live longer with HIV-1, non-Hodgkin's lymphoma (NHL) becomes an increasingly important clinical issue. At present, it is the second most common malignancy in HIV-infected adult patients. Approximately 10% (now over 3.5 million worldwide) of HIV-infected patients develop lymphoma. Unfortunately, therapies that would normally be used to combat NHL – chemotherapy, radiation and monoclonal antibodies – can actually hasten progression of HIV disease by exacerbating underlying immunosuppression. Highly active antiretroviral therapy (HAART), which is clearly invaluable, does not halt growth or proliferation of NHL, nor does it offer a definitive cure for HIV. Patients live longer, only to succumb to a malignancy for which few weapons are available. This gap in our clinical armamentarium calls for the development of innovative therapies.
We are interested in developing innovative therapies for HIV-infected patients with lymphoma (Fig. 3), which would provide proof-of-concept for similar multipronged immunotherapy in other disease states where tumors accompany viral infection, such as HBV/HCV and HCC and other virus-related malignancies.

Fig. 3: Overview of adoptive bi-specific CAR-T and CAR-NK cell therapy with HIV Patients with lymphoma These proposed in vitro experiments set the stage for future in vivo studies.
Project #3: Chimeric antigen receptors (CAR) immunological synapse
Recent clinical trials testing cancer immunotherapies have shown promising results for treating various cancers. One such therapy involves engineering immune cells to express chimeric antigen receptors (CAR), which combine tumor antigen specificity with immune cell activation in a single receptor. The adoptive transfer of these CAR-modified immune cells (especially T-cells, CAR T) into patients has shown remarkable success in treating multiple blood cancers.
To work well, CAR T-cells must form an effective immunological synapse (IS) with their susceptible tumor cells (Fig. 4). The CAR-modified T or NK cell IS is important to the understanding of CAR-modified NK cell-mediated cytotoxicity and side effects (Liu, D., et al., Immunity, 2009 and Liu D., et al., Protein & Cell, 2017).

Fig. 4: The CAR-NK cell Immunological Synapse. One CAR-modified NK92 cell (blue) interacts with a tumor cell (yellow) through an IS. Modified from Liu D. et al. Protein & Cell, 2017.
This project pioneers measuring the quality of the CAR T-cell IS using the glass supported planar lipid bilayer system (Fig. 5) to predict antitumor activity and will lead to the development of fast and easy tools to predict CAR T-cell effectiveness in cancer patients (Xiong et al., Molecular Therapy, 2018, and Liu et al., Cell Commun Signal, 2020). This project will test the hypothesis that the IS quality will predict CAR-modified cell efficacy (No Synapse, No Killing), which will correlate with clinical outcomes.
The long-term goals of this project are to develop fast and easy tools to predict CAR-modified cell efficacy/toxicity and to provide guidelines for designing and optimizing CARs for clinical therapy.

Fig. 5. A diagram of lipid bilayer. IgG1 kappa is a sample used to stimulate kappa.CAR T or NK cells on lipid bilayer. Modified from Kaizuka Y. et al. PNAS, 2007.
In summary, we are using molecular, biochemical, bioinformatics, immunological, and advanced super-resolution imaging strategies, as well as machine learning approaches, for understanding human lymphocyte (including CTLs and NK cells) dysfunction in chronic infectious diseases (e.g., HIV, HBV, and HPV) and cancers.

